LBF20406AM09: Difference between revisions
No edit summary |
No edit summary |
||
| (14 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
{{Metabolite | {{Metabolite | ||
|LipidBank=XPR7025 | |LipidBank=XPR7025 | ||
|LipidMaps=LMFA08020011 | |LipidMaps=LMFA08020011 | ||
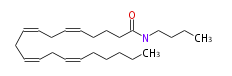
|SysName=N- | |SysName=N-Butyl- (cis-5,cis-8,cis-11,cis-14) -eicosatetraenoylamine | ||
|Common Name=&&N- | |Common Name=&&N-Butylarachidonoylamide&&N-Butyl- (5Z,8Z,11Z,14Z) -eicosatetraenoylamine&& | ||
|Melting Point=colorless oil [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |Melting Point=colorless oil [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | ||
|NMR Spectra= | |NMR Spectra=^1 H NMR (CDCl3) delta 5.26-5.44 (m, 8H), 3.24 (q, J=6Hz, 2H), 2.76-2.86 (m, 6H), 2.02-2.22(m, 6H), 1.64-1.78 (m, 2H), 1.22-1.56 (m, 10H), 0.86-0.98 (m, 6H). [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | ||
|Source= | |||
|Chemical Synthesis=This compound was synthesized from arachidonoylchloride and n-butylamine. Yield is 56%. [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |||
|Metabolism= | |||
|Biological Activity=Binding of this compound to the brain cannabinoid receptor (CB1),Ki (nM)= 235.7±l4.2 [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 04:41, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR7025 |
| LipidMaps | LMFA08020011 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406AM09 |
| N-Butylarachidonoylamide | |
|---|---|
| |
| Structural Information | |
| N-Butyl- (cis-5,cis-8,cis-11,cis-14) -eicosatetraenoylamine | |
| |
| Formula | C24H41NO |
| Exact Mass | 359.318814939 |
| Average Mass | 359.58847999999995 |
| SMILES | C(=CCCCC(NCCCC)=O)CC=CCC=CCC=CCCCCC |
| Physicochemical Information | |
| colorless oil Sheskin_T et al. | |
| This compound was synthesized from arachidonoylchloride and n-butylamine. Yield is 56%. Sheskin_T et al. | |
| Binding of this compound to the brain cannabinoid receptor (CB1),Ki (nM)= 235.7±l4.2 Sheskin_T et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 1H NMR (CDCl3) δ 5.26-5.44 (m, 8H), 3.24 (q, J=6Hz, 2H), 2.76-2.86 (m, 6H), 2.02-2.22(m, 6H), 1.64-1.78 (m, 2H), 1.22-1.56 (m, 10H), 0.86-0.98 (m, 6H). SheskinTet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|