LBF20406CV05: Difference between revisions
No edit summary |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 15: | Line 15: | ||
|NOTE Spectra=CD lambda _{ext}(EtOH)( Delta epsilon )nm 248(+3.8),291(-5.0).[[Reference:Kikuchi_H:Tsukitani_Y:Iguchi_K:Yamada_Y:,Tennen Yuki Kagoubutsu Toronkai Koen Yoshishu 26th,1983,26,220|{{RelationTable/GetFirstAuthor|Reference:Kikuchi_H:Tsukitani_Y:Iguchi_K:Yamada_Y:,Tennen Yuki Kagoubutsu Toronkai Koen Yoshishu 26th,1983,26,220}}]] | |NOTE Spectra=CD lambda _{ext}(EtOH)( Delta epsilon )nm 248(+3.8),291(-5.0).[[Reference:Kikuchi_H:Tsukitani_Y:Iguchi_K:Yamada_Y:,Tennen Yuki Kagoubutsu Toronkai Koen Yoshishu 26th,1983,26,220|{{RelationTable/GetFirstAuthor|Reference:Kikuchi_H:Tsukitani_Y:Iguchi_K:Yamada_Y:,Tennen Yuki Kagoubutsu Toronkai Koen Yoshishu 26th,1983,26,220}}]] | ||
|Source=Clavulones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard.[[Reference:Kikuchi_H:Tsukitani_Y:Iguchi_K:Yamada_Y:,Tetrahedron Lett.,1982,23,5171|{{RelationTable/GetFirstAuthor|Reference:Kikuchi_H:Tsukitani_Y:Iguchi_K:Yamada_Y:,Tetrahedron Lett.,1982,23,5171}}]][[Reference:Kobayashi_M:Yasuzawa_T:Yoshihara_M:Akutsu_H:Kyogoku_Y:Kitagawa_I:,Tetrahedron Lett.,1982,23,5331|{{RelationTable/GetFirstAuthor|Reference:Kobayashi_M:Yasuzawa_T:Yoshihara_M:Akutsu_H:Kyogoku_Y:Kitagawa_I:,Tetrahedron Lett.,1982,23,5331}}]][[Reference:Kikuchi_H:Tsukitani_Y:Iguchi_K:Yamada_Y:,Tetrahedron Lett.,1983,24,1549|{{RelationTable/GetFirstAuthor|Reference:Kikuchi_H:Tsukitani_Y:Iguchi_K:Yamada_Y:,Tetrahedron Lett.,1983,24,1549}}]][[Reference:Kobayashi_M:Yasuzawa_T:Yoshihara_M:Son_BW:Kyogoku_Y:Kitagawa_I:,Chem. Pharm. Bull. (Tokyo),1983,31,1440|{{RelationTable/GetFirstAuthor|Reference:Kobayashi_M:Yasuzawa_T:Yoshihara_M:Son_BW:Kyogoku_Y:Kitagawa_I:,Chem. Pharm. Bull. (Tokyo),1983,31,1440}}]] | |Source=Clavulones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard.[[Reference:Kikuchi_H:Tsukitani_Y:Iguchi_K:Yamada_Y:,Tetrahedron Lett.,1982,23,5171|{{RelationTable/GetFirstAuthor|Reference:Kikuchi_H:Tsukitani_Y:Iguchi_K:Yamada_Y:,Tetrahedron Lett.,1982,23,5171}}]][[Reference:Kobayashi_M:Yasuzawa_T:Yoshihara_M:Akutsu_H:Kyogoku_Y:Kitagawa_I:,Tetrahedron Lett.,1982,23,5331|{{RelationTable/GetFirstAuthor|Reference:Kobayashi_M:Yasuzawa_T:Yoshihara_M:Akutsu_H:Kyogoku_Y:Kitagawa_I:,Tetrahedron Lett.,1982,23,5331}}]][[Reference:Kikuchi_H:Tsukitani_Y:Iguchi_K:Yamada_Y:,Tetrahedron Lett.,1983,24,1549|{{RelationTable/GetFirstAuthor|Reference:Kikuchi_H:Tsukitani_Y:Iguchi_K:Yamada_Y:,Tetrahedron Lett.,1983,24,1549}}]][[Reference:Kobayashi_M:Yasuzawa_T:Yoshihara_M:Son_BW:Kyogoku_Y:Kitagawa_I:,Chem. Pharm. Bull. (Tokyo),1983,31,1440|{{RelationTable/GetFirstAuthor|Reference:Kobayashi_M:Yasuzawa_T:Yoshihara_M:Son_BW:Kyogoku_Y:Kitagawa_I:,Chem. Pharm. Bull. (Tokyo),1983,31,1440}}]] | ||
|Chemical Synthesis=Clavulone I was synthesized from cyclopentadiene and methyl 3-chloroformylpropionate as a racemic form.[[Reference:Corey_EJ:Mehrotra_MM:,J. Am. Chem. Soc.,1984,106,3384|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Mehrotra_MM:,J. Am. Chem. Soc.,1984,106,3384}}]]Clavulones were synthesized from L-(+)-diethyl tartarate and D-mannitol as a natural form.[[Reference:Nagaoka_H:Miyakoshi_T:Yamada_Y:,Tetrahedron Lett.,1984,25,3621|{{RelationTable/GetFirstAuthor|Reference:Nagaoka_H:Miyakoshi_T:Yamada_Y:,Tetrahedron Lett.,1984,25,3621}}]]Other synthesis of clavulone.[[Reference:Klunder_AJH:Zwanenburg_B:Liu_ZY:,Tetrahedron Lett.,1991,32,3131|{{RelationTable/GetFirstAuthor|Reference:Klunder_AJH:Zwanenburg_B:Liu_ZY:,Tetrahedron Lett.,1991,32,3131}}]][ | |Chemical Synthesis=Clavulone I was synthesized from cyclopentadiene and methyl 3-chloroformylpropionate as a racemic form.[[Reference:Corey_EJ:Mehrotra_MM:,J. Am. Chem. Soc.,1984,106,3384|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Mehrotra_MM:,J. Am. Chem. Soc.,1984,106,3384}}]]Clavulones were synthesized from L-(+)-diethyl tartarate and D-mannitol as a natural form.[[Reference:Nagaoka_H:Miyakoshi_T:Yamada_Y:,Tetrahedron Lett.,1984,25,3621|{{RelationTable/GetFirstAuthor|Reference:Nagaoka_H:Miyakoshi_T:Yamada_Y:,Tetrahedron Lett.,1984,25,3621}}]] Other synthesis of clavulone.[[Reference:Klunder_AJH:Zwanenburg_B:Liu_ZY:,Tetrahedron Lett.,1991,32,3131|{{RelationTable/GetFirstAuthor|Reference:Klunder_AJH:Zwanenburg_B:Liu_ZY:,Tetrahedron Lett.,1991,32,3131}}]] | ||
[Reference:Takemoto M:Koshida:A:Miyazima K:Suzuki K:Achiwa K:,Chem. Pharm. Bull. (Tokyo),1991,39,1106|{{RelationTable/GetFirstAuthor|Reference:Takemoto M:Koshida:A:Miyazima K:Suzuki K:Achiwa K:,Chem. Pharm. Bull. (Tokyo),1991,39,1106}}]] | [[Reference:Takemoto M:Koshida:A:Miyazima K:Suzuki K:Achiwa K:,Chem. Pharm. Bull. (Tokyo),1991,39,1106|{{RelationTable/GetFirstAuthor|Reference:Takemoto M:Koshida:A:Miyazima K:Suzuki K:Achiwa K:,Chem. Pharm. Bull. (Tokyo),1991,39,1106}}]] | ||
[[Reference:Zhu_J:Yang_JY:Klunder_AJH:Liu_ZY:Zwanenburg_B:,Tetrahedron,1995,51,5847|{{RelationTable/GetFirstAuthor|Reference:Zhu_J:Yang_JY:Klunder_AJH:Liu_ZY:Zwanenburg_B:,Tetrahedron,1995,51,5847}}]] | [[Reference:Zhu_J:Yang_JY:Klunder_AJH:Liu_ZY:Zwanenburg_B:,Tetrahedron,1995,51,5847|{{RelationTable/GetFirstAuthor|Reference:Zhu_J:Yang_JY:Klunder_AJH:Liu_ZY:Zwanenburg_B:,Tetrahedron,1995,51,5847}}]] | ||
|Metabolism=The biosynthesis of clavulone is suggested to proceed from arachidonic acid via 8(R)-HEPETE and pre-clavulone A.[[Reference:Corey_EJ:,Experientia,1983,39,1084|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:,Experientia,1983,39,1084}}]][[Reference:Corey_EJ:Schmidt_G:Shimoji_K:,Tetrahedron Lett.,1983,24,3169|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Schmidt_G:Shimoji_K:,Tetrahedron Lett.,1983,24,3169}}]][[Reference:Corey_EJ:Lansbury_PT_Jr:Yamada_Y:,Tetrahedron Lett.,1985,26,4171|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Lansbury_PT_Jr:Yamada_Y:,Tetrahedron Lett.,1985,26,4171}}]][[Reference:Corey_EJ:dAlarcao_M:Matsuda_SPT:Lansbury_PT_Jr:Yamada_Y:,J. Am. Chem. Soc.,1987,109,289|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:dAlarcao_M:Matsuda_SPT:Lansbury_PT_Jr:Yamada_Y:,J. Am. Chem. Soc.,1987,109,289}}]] | |Metabolism=The biosynthesis of clavulone is suggested to proceed from arachidonic acid via 8(R)-HEPETE and pre-clavulone A.[[Reference:Corey_EJ:,Experientia,1983,39,1084|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:,Experientia,1983,39,1084}}]][[Reference:Corey_EJ:Schmidt_G:Shimoji_K:,Tetrahedron Lett.,1983,24,3169|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Schmidt_G:Shimoji_K:,Tetrahedron Lett.,1983,24,3169}}]][[Reference:Corey_EJ:Lansbury_PT_Jr:Yamada_Y:,Tetrahedron Lett.,1985,26,4171|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Lansbury_PT_Jr:Yamada_Y:,Tetrahedron Lett.,1985,26,4171}}]][[Reference:Corey_EJ:dAlarcao_M:Matsuda_SPT:Lansbury_PT_Jr:Yamada_Y:,J. Am. Chem. Soc.,1987,109,289|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:dAlarcao_M:Matsuda_SPT:Lansbury_PT_Jr:Yamada_Y:,J. Am. Chem. Soc.,1987,109,289}}]] | ||
Latest revision as of 10:37, 12 November 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR8001 |
| LipidMaps | LMFA03120001 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406CV05 |
| Clavulone I | |
|---|---|
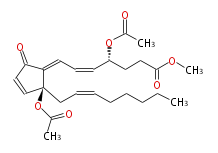
| |
| Structural Information | |
| Methyl-4R- (cis-5,trans-7) -4-acetoxy-7- [ 2S-acetoxy-2- (cis-2-octenyl) -5-oxo-3-cyclopentenylidene] -5-heptenoic acid | |
| |
| Formula | C25H34O7 |
| Exact Mass | 446.230453442 |
| Average Mass | 446.53326000000004 |
| SMILES | O(C(C)=O)[C@@](C1=CC=C[C@@H](CCC(OC)=O)OC(C)=O)(CC=CCCCCC)C=CC1=O |
| Physicochemical Information | |
| [ α ]D -28.9°(C 0.36, CHCl3) KikuchiHet al. | |
| Clavulones are soluble in MeOH, EtOH, CHCl3 or hexane. | |
| Clavulones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard. Kikuchi_H et al. Kobayashi_M et al. Kikuchi_H et al. Kobayashi_M et al. | |
| Clavulone I was synthesized from cyclopentadiene and methyl 3-chloroformylpropionate as a racemic form. Corey_EJ et al.Clavulones were synthesized from L-(+)-diethyl tartarate and D-mannitol as a natural form. Nagaoka_H et al. Other synthesis of clavulone. Klunder_AJH et al. | |
| The biosynthesis of clavulone is suggested to proceed from arachidonic acid via 8(R)-HEPETE and pre-clavulone A. Corey_EJ Corey_EJ et al. Corey_EJ et al. Corey_EJ et al. | |
| Clavulones showed a significant anti-inflammatory effect at 30 mu g/ml by the fertile egg test. Kikuchi_H et al.Clavulone I showed strong antiproliferative activity in the human myeloid leukemia (HL-60) cells (IC_{50} 0.2 mu g/ml). Clavulone arrests the cells in the G1-phase and inhibits the cell growth of HL-60 cells by inhibiting S-phase DNA synthesis. Honda_A et al.Clavulone showed positive chronotropic action on the cultured myocardial cells. Honda_A et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max(EtOH) 230 nm( ε 13600),292 nm( ε 17300) KikuchiHet al. |
| IR Spectra | ν max(film)1730,1700,1635,and 1230cm-1 KikuchiHet al. |
| NMR Spectra | 1H-NMR(270MHz,CDCl3) δ ppm0.88(3H,t,J=6.7Hz),2.03(3H,s),2.05(3H,s),2.38(2H,t,J=7.7Hz),2.66(1H,dd,J=7,14.5Hz),2.97(1H,dd,J=7,14.5Hz),3.70(3H,s),5.22(1H,dt,J=10.9,7Hz),5.45(1H,dt,J=10.9,8Hz),5.78(1H,m),5.86(1H,t,J=10Hz),6.42(1H,d,J=6.3Hz),6.59(1H,dd,J=10,12.5Hz),7.25(1H,d,J=12.5Hz),7.47(1H,d,J=6.3Hz). KikuchiHet al.13C-NMR(67.8MHz,CDCl3) δ ppm14.0(q),20.9(q),21.2(q),22.5(t),27.4(t),29.0(t),29.8(t),29.8(t),31.4(t),35.9(t),51.7(q),69.4(d),85.2(s),121.0(d),124.3(d),124.4(d),134.9(d),137.5(s),138.7(d),157.8(d),169.0(s),169.7(s),172.7(s),193.0(s). KikuchiHet al. |
| Other Spectra | CD λ ext(EtOH)( Δ ε )nm 248(+3.8),291(-5.0). KikuchiHet al. |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|