LBF20406CV12
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR8008 |
| LipidMaps | LMFA03120008 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406CV12 |
| Chlorovulone I | |
|---|---|
| |
| Structural Information | |
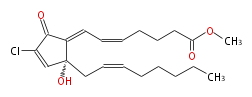
| Methyl- (5-cis,7-trans) -7- [ 4R-chloro-2-hydroxy-2- [cis-2-octenyl] -5-oxo-3-cyclopentenylidene ] -5-heptenoic acid | |
| |
| Formula | C21H29ClO4 |
| Exact Mass | 380.175437126 |
| Average Mass | 380.90525999999994 |
| SMILES | C(C(=O)1)(Cl)=C[C@](CC=CCCCCC)(C(=CC=CCCCC(OC)=O)1)O |
| Physicochemical Information | |
| [ α ]D -1.2°(C 0.17, CHCl3) IguchiKet al. | |
| Chlorovulones are soluble in MeOH, EtOH, CHCl3, or hexane. | |
| Chlorovulones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard. Iguchi_K et al. Nagaoka_H et al. | |
| Photoisomerization of chlorovulone I (fluoresent lamp, benzene, 40 hr) gave a mixture of chlorovulones I, II and III. Iguchi_K et al. | |
| Chlorovulone I showed the strong antiproliferative and cytotoxic activities in himan promyelocytic leukemia (HL-60) ((IC_{50} 0.01 mu g/ml, cytotoxic effect >0.1 mu g/ml). Honda_A et al. Honda_A et al. Iguchi_K et al.Chlorovulone I was entrapped into lipid microspheres of 0.2 mu m diameter to lipo-drug. Daily treatment with lipo-chlorovulone I (1.6 mg/kg/day, i.p.) on days 1 through 5 markedly prolonged the survival time (135% ILS) of mice inoculated with sarcoma 180 as compared with that of corresponding dose of respective free chlorovulone I. Honda_A et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max(EtOH) 243 nm( ε 14600),315 nm( ε 15100) IguchiKet al. |
| IR Spectra | ν max(CHCl3)1705cm-1 IguchiKet al. |
| NMR Spectra | 1H-NMR(400MHz,CDCl3) δ ppm0.88(3H,t,J=7.2Hz),1.30(6H,m),1.80(2H,quint.,J=7.4Hz),1.97(2H,brq,J=7.0Hz),2.35(2H,t,J=7.4Hz),2.42(2H,m),2.67(1H,ddd,J=0.5,7.8,14.2Hz),2.82(1H,ddd,J=0.5,7.5,14.2Hz),3.68(3H,s),5.22(1H,ttd,J=1.5,7.7,10.9Hz),5.54(1H,ttd,J=1.4,8.7,10.9Hz),6.11(1H,tdd,J=7.9,0.9,10.9Hz),6.77(1H,tdd,J=1.5,10.9,12.6Hz),7.21(1H,d,J=0.6Hz),7.33(1H,brd,J=12.6Hz). IguchiKet al.13C-NMR(100MHz,CDCl3) δ ppm13.9(q),22.4(t),24.3(t),27.1(t),27.3(t),29.0(t),31.4(t),33.2(t),33.6(t),51.6(q),77.7(s),121.7(d),123.8(d),127.9(d),134.8(d),136.4(s),137.9(s),143.6(d),154.0(d),173.6(s),187.7(s). IguchiKet al. |
| Other Spectra | CD λ ext(EtOH)( Δ ε )nm 232(+7.6),265(-6.1),360(+3.1). IguchiKet al. |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|