LBF20406HP01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 11: | Line 11: | ||
|UV Spectra=UV[[Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177}}]]conjugated diene: <FONT FACE="Symbol">l</FONT>max=235nm, UV(Me-ester)[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587}}]] conjugated diene: <FONT FACE="Symbol">l</FONT>max=232.5nm, UV(Me-ester; after reduction)[[Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058}}]][[Reference:Borgeat_P:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1976,251,7816|{{RelationTable/GetFirstAuthor|Reference:Borgeat_P:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1976,251,7816}}]] conjugated trans, cis diene: <FONT FACE="Symbol">l</FONT>max=235-236nm, conjugated trans, trans diene: <FONT FACE="Symbol">l</FONT>m | |UV Spectra=UV[[Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177}}]]conjugated diene: <FONT FACE="Symbol">l</FONT>max=235nm, UV(Me-ester)[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587}}]] conjugated diene: <FONT FACE="Symbol">l</FONT>max=232.5nm, UV(Me-ester; after reduction)[[Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058}}]][[Reference:Borgeat_P:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1976,251,7816|{{RelationTable/GetFirstAuthor|Reference:Borgeat_P:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1976,251,7816}}]] conjugated trans, cis diene: <FONT FACE="Symbol">l</FONT>max=235-236nm, conjugated trans, trans diene: <FONT FACE="Symbol">l</FONT>m | ||
|IR Spectra=IR(Me-ester; after reduction)[[Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058}}]]conjugated trans, cis diene: 985, 950cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>, conjugated trans, trans diene: 989cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | |IR Spectra=IR(Me-ester; after reduction)[[Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058}}]]conjugated trans, cis diene: 985, 950cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>, conjugated trans, trans diene: 989cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | ||
|Source=Autooxidation of arachidonic acid[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595}}]][[Reference:Yamagata_S:Murakami_H:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1983,47,2791|{{RelationTable/GetFirstAuthor|Reference:Yamagata_S:Murakami_H:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1983,47,2791}}]][[Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058}}]]. Oxidation of arachidonic acid by singlet-oxygen[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587}}]][[Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177}}]]. Reaction products between arachidonic acid and lipoxygenase from living cells(5-HPETE)[[Reference:Spector_AA:Gordon_JA:Moore_SA:,Prog. Lipid Res.,1988,27,271|{{RelationTable/GetFirstAuthor|Reference:Spector_AA:Gordon_JA:Moore_SA:,Prog. Lipid Res.,1988,27,271}}]]. | |Source=Autooxidation of arachidonic acid[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595}}]][[Reference:Yamagata_S:Murakami_H:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1983,47,2791|{{RelationTable/GetFirstAuthor|Reference:Yamagata_S:Murakami_H:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1983,47,2791}}]][[Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058}}]]. Oxidation of arachidonic acid by singlet-oxygen[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587}}]][[Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177}}]]. Reaction products between arachidonic acid and lipoxygenase from living cells(5-HPETE)<!--8031--><!--8100-->[[Reference:Spector_AA:Gordon_JA:Moore_SA:,Prog. Lipid Res.,1988,27,271|{{RelationTable/GetFirstAuthor|Reference:Spector_AA:Gordon_JA:Moore_SA:,Prog. Lipid Res.,1988,27,271}}]]<!--8102--><!--8103-->. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
|Biological Activity=5-HPETE generated by 5-lipoxygenase is enzymatically converted to bioactive compounds such as leukotriene and HETE[[Reference:Spector_AA:Gordon_JA:Moore_SA:,Prog. Lipid Res.,1988,27,271|{{RelationTable/GetFirstAuthor|Reference:Spector_AA:Gordon_JA:Moore_SA:,Prog. Lipid Res.,1988,27,271}}]]. | |Biological Activity=5-HPETE generated by 5-lipoxygenase is enzymatically converted to bioactive compounds such as leukotriene and HETE<!--8100-->[[Reference:Spector_AA:Gordon_JA:Moore_SA:,Prog. Lipid Res.,1988,27,271|{{RelationTable/GetFirstAuthor|Reference:Spector_AA:Gordon_JA:Moore_SA:,Prog. Lipid Res.,1988,27,271}}]]<!--8103--><!--8104-->. | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 16:30, 26 January 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8079 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406HP01 |
| 5-Hydroperoxy-6,8,11,14-Eicosatetraenoic Acid | |
|---|---|
| |
| Structural Information | |
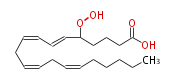
| 5-Hydroperoxy-6,8,11,14-Eicosatetraenoic Acid/5-Hydroperoxy-6,8,11,14-Eicosatetraenoate | |
| |
| Formula | C20H32O4 |
| Exact Mass | 336.23005951199997 |
| Average Mass | 336.46567999999996 |
| SMILES | C(CC=CCC=CCC=CC=CC(OO)CCCC(O)=O)CCC |
| Physicochemical Information | |
| Autooxidation of arachidonic acid Terao_J et al. Yamagata_S et al. Porter_NA et al.. Oxidation of arachidonic acid by singlet-oxygen Terao_J et al. Porter_NA et al.. Reaction products between arachidonic acid and lipoxygenase from living cells(5-HPETE) Spector_AA et al.. | |
| 5-HPETE generated by 5-lipoxygenase is enzymatically converted to bioactive compounds such as leukotriene and HETE Spector_AA et al.. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(Me-ester; after reduction and TMS) TeraoJet al. TeraoJet al. BorgeatPet al. RabinovitchHet al. KoshiharaYet al. OchiKet al. FurukawaMet al.GC-EI-MS(Me-ester; after reduction and TBDMS)(114), GC-EI-MS(Me-ester; after reduction, hydrogenation and TMS) TeraoJet al. TeraoJet al. Porter_NA et al. Porter_NA et al. BorgeatPet al. RabinovitchHet al. MayerBet al. |
| UV Spectra | UV Porter_NA et al.conjugated diene: lmax=235nm, UV(Me-ester) TeraoJet al. conjugated diene: lmax=232.5nm, UV(Me-ester; after reduction) Porter_NA et al. BorgeatPet al. conjugated trans, cis diene: lmax=235-236nm, conjugated trans, trans diene: lm |
| IR Spectra | IR(Me-ester; after reduction) Porter_NA et al.conjugated trans, cis diene: 985, 950cm-1, conjugated trans, trans diene: 989cm-1 |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|