LBF20406PH01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA08020002 | |LipidMaps=LMFA08020002 | ||
|SysName=anandamide 0-phosphate | |SysName=anandamide 0-phosphate | ||
|Common Name=&&anandamide 0-phosphate&& | |||
|Melting Point=colorless oil [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |Melting Point=colorless oil [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H NMR (CDCl3) <FONT FACE="Symbol">d</FONT>5.34-5.42 (m, 8H), 3.98 (br s 2H), 3.46 (br s 2H), 2.77-2.81 (m, 6H), 2.26 (t, J=6.8Hz, 2H), 2.00-2.06 (m, 4H), 1.60-1.69 (m, 2H), 1.29-1.42 (m, 6H), 0.88 (t, J=6.9Hz,3H) [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H NMR (CDCl3) <FONT FACE="Symbol">d</FONT>5.34-5.42 (m, 8H), 3.98 (br s 2H), 3.46 (br s 2H), 2.77-2.81 (m, 6H), 2.26 (t, J=6.8Hz, 2H), 2.00-2.06 (m, 4H), 1.60-1.69 (m, 2H), 1.29-1.42 (m, 6H), 0.88 (t, J=6.9Hz,3H) [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | ||
}} | }} | ||
Revision as of 00:01, 20 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR7016 |
| LipidMaps | LMFA08020002 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406PH01 |
| anandamide 0-phosphate | |
|---|---|
| |
| Structural Information | |
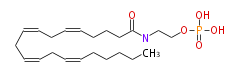
| anandamide 0-phosphate | |
| |
| Formula | C22H38NO5P |
| Exact Mass | 427.2487598409999 |
| Average Mass | 427.51462100000003 |
| SMILES | C(CCCC=CCC=CCC=CCC=CCCCCC)(NCCOP(O)(O)=O)=O |
| Physicochemical Information | |
| colorless oil Sheskin_T et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 1H NMR (CDCl3) d5.34-5.42 (m, 8H), 3.98 (br s 2H), 3.46 (br s 2H), 2.77-2.81 (m, 6H), 2.26 (t, J=6.8Hz, 2H), 2.00-2.06 (m, 4H), 1.60-1.69 (m, 2H), 1.29-1.42 (m, 6H), 0.88 (t, J=6.9Hz,3H) SheskinTet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|