LBF18108HO04: Difference between revisions
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
|LipidMaps=LMFA01050134 | |LipidMaps=LMFA01050134 | ||
|SysName=9,12,13-Trihydroxy-10-octadecenoic acid | |SysName=9,12,13-Trihydroxy-10-octadecenoic acid | ||
|Common Name=&&9,12,13-Trihydroxy-10-octadecenoate | |Common Name=&&9,12,13-Trihydroxy-10-octadecenoate&& | ||
|Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation)[[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]][[Reference:Schieberle_P:Tsoukalas_B:Grosch_W:,Z. Lebensm. Unters. Forsch.,1979,168,448|{{RelationTable/GetFirstAuthor|Reference:Schieberle_P:Tsoukalas_B:Grosch_W:,Z. Lebensm. Unters. Forsch.,1979,168,448}}]][[Reference:Gardner_HW:Nelson_EC:Tjarks_LW:England_RE:,Chem. Phys. Lipids,1984,35,87|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Nelson_EC:Tjarks_LW:England_RE:,Chem. Phys. Lipids,1984,35,87}}]][[Reference:Graveland_A_:,J. Am. Oil Chem. Soc.,1970,47,352|{{RelationTable/GetFirstAuthor|Reference:Graveland_A_:,J. Am. Oil Chem. Soc.,1970,47,352}}]][[Reference:Galliard_T:Phillips_DR:Matthew_JA:,Biochim. Biophys. Acta,1975,409,157|{{RelationTable/GetFirstAuthor|Reference:Galliard_T:Phillips_DR:Matthew_JA:,Biochim. Biophys. Acta,1975,409,157}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908}}]]: m/e=545[M-CH3], 529[M-OCH3], 460[rearrangment peak], 259[SMTO=CH-(CH2)7COOCH3], 173[SMTO=CH-(CH2)4CH3], 387[M-173], 301[M-259], 298[M-HOTMS], GC-EI-MS(after methanolysis, trimethylsilylation and isopropylidene treatment), GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation) | |Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation)[[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]][[Reference:Schieberle_P:Tsoukalas_B:Grosch_W:,Z. Lebensm. Unters. Forsch.,1979,168,448|{{RelationTable/GetFirstAuthor|Reference:Schieberle_P:Tsoukalas_B:Grosch_W:,Z. Lebensm. Unters. Forsch.,1979,168,448}}]][[Reference:Gardner_HW:Nelson_EC:Tjarks_LW:England_RE:,Chem. Phys. Lipids,1984,35,87|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Nelson_EC:Tjarks_LW:England_RE:,Chem. Phys. Lipids,1984,35,87}}]][[Reference:Graveland_A_:,J. Am. Oil Chem. Soc.,1970,47,352|{{RelationTable/GetFirstAuthor|Reference:Graveland_A_:,J. Am. Oil Chem. Soc.,1970,47,352}}]][[Reference:Galliard_T:Phillips_DR:Matthew_JA:,Biochim. Biophys. Acta,1975,409,157|{{RelationTable/GetFirstAuthor|Reference:Galliard_T:Phillips_DR:Matthew_JA:,Biochim. Biophys. Acta,1975,409,157}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908}}]]: m/e=545[M-CH3], 529[M-OCH3], 460[rearrangment peak], 259[SMTO=CH-(CH2)7COOCH3], 173[SMTO=CH-(CH2)4CH3], 387[M-173], 301[M-259], 298[M-HOTMS], GC-EI-MS(after methanolysis, trimethylsilylation and isopropylidene treatment), GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation) | ||
|IR Spectra=Methyl ester: olefinic trans unsaturation(990-965 cm^{-1}), free OH(3620-3595 cm^{-1}), bonded OH(3640-3160cm^{-1})[[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]][[Reference:Schieberle_P:Tsoukalas_B:Grosch_W:,Z. Lebensm. Unters. Forsch.,1979,168,448|{{RelationTable/GetFirstAuthor|Reference:Schieberle_P:Tsoukalas_B:Grosch_W:,Z. Lebensm. Unters. Forsch.,1979,168,448}}]][[Reference:Graveland_A_:,J. Am. Oil Chem. Soc.,1970,47,352|{{RelationTable/GetFirstAuthor|Reference:Graveland_A_:,J. Am. Oil Chem. Soc.,1970,47,352}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]] | |IR Spectra=Methyl ester: olefinic trans unsaturation(990-965 cm^{-1}), free OH(3620-3595 cm^{-1}), bonded OH(3640-3160cm^{-1})[[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]][[Reference:Schieberle_P:Tsoukalas_B:Grosch_W:,Z. Lebensm. Unters. Forsch.,1979,168,448|{{RelationTable/GetFirstAuthor|Reference:Schieberle_P:Tsoukalas_B:Grosch_W:,Z. Lebensm. Unters. Forsch.,1979,168,448}}]][[Reference:Graveland_A_:,J. Am. Oil Chem. Soc.,1970,47,352|{{RelationTable/GetFirstAuthor|Reference:Graveland_A_:,J. Am. Oil Chem. Soc.,1970,47,352}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]] | ||
Revision as of 21:00, 14 April 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8032 |
| LipidMaps | LMFA01050134 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18108HO04 |
| 9,12,13-Trihydroxy-10-octadecenoate | |
|---|---|
| |
| Structural Information | |
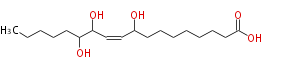
| 9,12,13-Trihydroxy-10-octadecenoic acid | |
| |
| Formula | C18H34O5 |
| Exact Mass | 330.240624198 |
| Average Mass | 330.45956 |
| SMILES | CCCCCC(O)C(O)C=CC(O)CCCCCCCC(O)=O |
| Physicochemical Information | |
| Reaction products between hydroperoxylinoleate and soy bean lipoxygenase Streckert_G et al. or potato extracts Galliard_T et al.. Major reactive products between 13-hydroperoxylinoleate and hemathin Dix_TA et al.. Oxidative products of 13-hydroperoxylinoleate Gardner_HW et al.. A degradation product of 13-hydroperoxylinoleate in the presence of Fe(III)-cystein Gardner_HW et al.. | |
| It showed a toxicity corresponding to linoleate monohydroxyperoxide Fujimoto_K . | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after methanolysis and trimethylsilylation) StreckertGet al. Neff_WE et al. SchieberlePet al. Gardner_HW et al. Graveland_A_ GalliardTet al. Frankel_EN et al.: m/e=545[M-CH3], 529[M-OCH3], 460[rearrangment peak], 259[SMTO=CH-(CH2)7COOCH3], 173[SMTO=CH-(CH2)4CH3], 387[M-173], 301[M-259], 298[M-HOTMS], GC-EI-MS(after methanolysis, trimethylsilylation and isopropylidene treatment), GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation) |
| UV Spectra | |
| IR Spectra | Methyl ester: olefinic trans unsaturation(990-965 cm-1), free OH(3620-3595 cm-1), bonded OH(3640-3160cm-1) StreckertGet al. Neff_WE et al. SchieberlePet al. Graveland_A_ Sessa_DJ et al. |
| NMR Spectra | 1H-NMR(methyl ester) Neff_WE et al. Gardner_HW et al. Graveland_A_ :olefinic protons(5.74-5.86ppm), C9, 12(3.7-4.2ppm), C13(3.2-3.77ppm), OH(3.6ppm) |
| Other Spectra | ODR analysis Gardner_HW et al. |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|