LBF18206HP01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
|IR Spectra=Methyl ester: [[Reference:Chan_HW:Levett_G:,Lipids,1977,12,99|{{RelationTable/GetFirstAuthor|Reference:Chan_HW:Levett_G:,Lipids,1977,12,99}}]][[Reference:Gardner_HW:Weisleder_D:,Lipids,1970,5,678|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1970,5,678}}]][[Reference:Cannon_JA:Zilch_KT:Burket_SC:Dutton_HJ:,J. Am. Oil Chem. Soc.,1952,29,447|{{RelationTable/GetFirstAuthor|Reference:Cannon_JA:Zilch_KT:Burket_SC:Dutton_HJ:,J. Am. Oil Chem. Soc.,1952,29,447}}]][[Reference:Privett_OS:Lundberg_WO:Khan_NA:Tolberg_WE:Wheeler_DH:,J. Am. Oil Chem. Soc.,1953,30,61|{{RelationTable/GetFirstAuthor|Reference:Privett_OS:Lundberg_WO:Khan_NA:Tolberg_WE:Wheeler_DH:,J. Am. Oil Chem. Soc.,1953,30,61}}]][[Reference:Sephton_HH:Sutton_DA:,J. Am. Oil Chem. Soc.,1956,33,263|{{RelationTable/GetFirstAuthor|Reference:Sephton_HH:Sutton_DA:,J. Am. Oil Chem. Soc.,1956,33,263}}]][[Reference:Graveland_A_:,J. Am. Oil Chem. Soc.,1970,47,352|{{RelationTable/GetFirstAuthor|Reference:Graveland_A_:,J. Am. Oil Chem. Soc.,1970,47,352}}]][[Reference:Privett_OS:Nickell_C:Lundberg_WO:Boyer_PD:,J. Am. Oil Chem. Soc.,1955,32,505|{{RelationTable/GetFirstAuthor|Reference:Privett_OS:Nickell_C:Lundberg_WO:Boyer_PD:,J. Am. Oil Chem. Soc.,1955,32,505}}]][[Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191}}]]: trans, cis isomer: 986 and 949cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>, trans, trans isomer: 989cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>, OOH group: 3550cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | |IR Spectra=Methyl ester: [[Reference:Chan_HW:Levett_G:,Lipids,1977,12,99|{{RelationTable/GetFirstAuthor|Reference:Chan_HW:Levett_G:,Lipids,1977,12,99}}]][[Reference:Gardner_HW:Weisleder_D:,Lipids,1970,5,678|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1970,5,678}}]][[Reference:Cannon_JA:Zilch_KT:Burket_SC:Dutton_HJ:,J. Am. Oil Chem. Soc.,1952,29,447|{{RelationTable/GetFirstAuthor|Reference:Cannon_JA:Zilch_KT:Burket_SC:Dutton_HJ:,J. Am. Oil Chem. Soc.,1952,29,447}}]][[Reference:Privett_OS:Lundberg_WO:Khan_NA:Tolberg_WE:Wheeler_DH:,J. Am. Oil Chem. Soc.,1953,30,61|{{RelationTable/GetFirstAuthor|Reference:Privett_OS:Lundberg_WO:Khan_NA:Tolberg_WE:Wheeler_DH:,J. Am. Oil Chem. Soc.,1953,30,61}}]][[Reference:Sephton_HH:Sutton_DA:,J. Am. Oil Chem. Soc.,1956,33,263|{{RelationTable/GetFirstAuthor|Reference:Sephton_HH:Sutton_DA:,J. Am. Oil Chem. Soc.,1956,33,263}}]][[Reference:Graveland_A_:,J. Am. Oil Chem. Soc.,1970,47,352|{{RelationTable/GetFirstAuthor|Reference:Graveland_A_:,J. Am. Oil Chem. Soc.,1970,47,352}}]][[Reference:Privett_OS:Nickell_C:Lundberg_WO:Boyer_PD:,J. Am. Oil Chem. Soc.,1955,32,505|{{RelationTable/GetFirstAuthor|Reference:Privett_OS:Nickell_C:Lundberg_WO:Boyer_PD:,J. Am. Oil Chem. Soc.,1955,32,505}}]][[Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191}}]]: trans, cis isomer: 986 and 949cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>, trans, trans isomer: 989cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>, OOH group: 3550cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR[[Reference:Chan_HW:Levett_G:,Lipids,1977,12,99|{{RelationTable/GetFirstAuthor|Reference:Chan_HW:Levett_G:,Lipids,1977,12,99}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908}}]], <SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR( after methanolyzation and reduction)[[Reference:Gardner_HW:Weisleder_D:,Lipids,1970,5,678|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1970,5,678}}]][[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]]: trans,cis isomer: C10-13 (5.42-6.48ppm), C14 (2.10-2.18ppm), C9(4.15ppm), J10-11= 15.4Hz(trans), J12-13= 10.8Hz (cis), trans, trans isomer: olefinic protons (5.41ppm), C14 (2.07ppm), C9 (4.20ppm) | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR[[Reference:Chan_HW:Levett_G:,Lipids,1977,12,99|{{RelationTable/GetFirstAuthor|Reference:Chan_HW:Levett_G:,Lipids,1977,12,99}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908}}]], <SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR( after methanolyzation and reduction)[[Reference:Gardner_HW:Weisleder_D:,Lipids,1970,5,678|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1970,5,678}}]][[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]]: trans,cis isomer: C10-13 (5.42-6.48ppm), C14 (2.10-2.18ppm), C9(4.15ppm), J10-11= 15.4Hz(trans), J12-13= 10.8Hz (cis), trans, trans isomer: olefinic protons (5.41ppm), C14 (2.07ppm), C9 (4.20ppm) | ||
|Source=Auto oxidation of methyllinoleate[[Reference:Frankel_EN:,Prog. Lipid Res.,1980,19,1|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1980,19,1}}]][[Reference:Frankel_EN:,Prog. Lipid Res.,1983,22,1|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1983,22,1}}]][[Reference:Frankel_EN:,Prog. Lipid Res.,1984,23,197|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1984,23,197}}]][[Reference:Frankel_EN:,Chem. Phys. Lipids,1987,44,73|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Chem. Phys. Lipids,1987,44,73}}]][[Reference:Chan_HWS:Coxon_DT:Peers_KE:Price_KR:,Food Chemistry,1982,9,21|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:Coxon_DT:Peers_KE:Price_KR:,Food Chemistry,1982,9,21}}]] | |Source=Auto oxidation of methyllinoleate[[Reference:Frankel_EN:,Prog. Lipid Res.,1980,19,1|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1980,19,1}}]][[Reference:Frankel_EN:,Prog. Lipid Res.,1983,22,1|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1983,22,1}}]][[Reference:Frankel_EN:,Prog. Lipid Res.,1984,23,197|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1984,23,197}}]][[Reference:Frankel_EN:,Chem. Phys. Lipids,1987,44,73|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Chem. Phys. Lipids,1987,44,73}}]][[Reference:Chan_HWS:Coxon_DT:Peers_KE:Price_KR:,Food Chemistry,1982,9,21|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:Coxon_DT:Peers_KE:Price_KR:,Food Chemistry,1982,9,21}}]]. Oxidation of methyl linoleate by singlet oxygen[[Reference:Frankel_EN:,Prog. Lipid Res.,1983,22,1|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1983,22,1}}]][[Reference:Frankel_EN:,Prog. Lipid Res.,1984,23,197|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1984,23,197}}]][[Reference:Frankel_EN:,Chem. Phys. Lipids,1987,44,73|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Chem. Phys. Lipids,1987,44,73}}]][[Reference:Frankel_EN:,Prog. Lipid Res.,1980,19,1|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1980,19,1}}]]. Oxidation of linoleic acid by lipoxygenase[[Reference:Mathuo_M:,Fragrance J. (in Japanese),1986,76,15|{{RelationTable/GetFirstAuthor|Reference:Mathuo_M:,Fragrance J. (in Japanese),1986,76,15}}]][[Reference:Wakabayashi_T:,Chemistry and Biology (in Japanese),1980,18,558|{{RelationTable/GetFirstAuthor|Reference:Wakabayashi_T:,Chemistry and Biology (in Japanese),1980,18,558}}]]. Production mechanism (auto oxidation): bis-allylic hydrogen at C11. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
Revision as of 11:55, 25 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8001 |
| LipidMaps | LMFA01040004 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18206HP01 |
| 9-Hydroperoxy-10,12-Octadecadienoic Acid | |
|---|---|
| |
| Structural Information | |
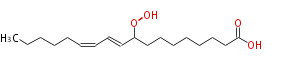
| 9-Hydroperoxy-10,12-Octadecadienoic Acid/9-Hydroperoxy-10,12-Octadecadienoate | |
| |
| Formula | C18H32O4 |
| Exact Mass | 312.23005951199997 |
| Average Mass | 312.44428 |
| SMILES | CCCCCC=CC=CC(OO)CCCCCCCC(O)=O |
| Physicochemical Information | |
| Auto oxidation of methyllinoleate Frankel_EN Frankel_EN Frankel_EN Frankel_EN Chan_HWS et al.. Oxidation of methyl linoleate by singlet oxygen Frankel_EN Frankel_EN Frankel_EN Frankel_EN . Oxidation of linoleic acid by lipoxygenase Mathuo_M Wakabayashi_T . Production mechanism (auto oxidation): bis-allylic hydrogen at C11. | |
| Spectral Information | |
| Mass Spectra | GC/EI-MS(after methanolysis, reduction and trimethylsilylation) Frankel_EN et al. KleimanRet al. Gardner_HW et al. Frankel_EN et al. HambergM: m/e= 382[M], 292[M-HOTMS], 311[M-(CH2)4CH3], 225[M-(CH2)7COOCH3] standard peak/ GC-EI-MS(after methylation, reduction and hydrogenation) Chan_HWS DolevAet al. Zimmerman_DC et al.: m/e= 187[CH(OH)(CH2)7COOCH3], 158[(CH2)7COOCH3+H], 155[C(OH)-(CH)7CO] |
| UV Spectra | Trans, cis isomer: l max=236nm, e =25900, trans, trans isomer: l max=233nm, e=28600 Chan_HW et al. Bolland_JL et al. Lundberg_WO et al. Lundberg_WO et al. Gardner_HW et al. Gardner_HW et al. |
| IR Spectra | Methyl ester: Chan_HW et al. Gardner_HW et al. Cannon_JA et al. Privett_OS et al. Sephton_HH et al. Graveland_A_ Privett_OS et al. Gardner_HW et al.: trans, cis isomer: 986 and 949cm-1, trans, trans isomer: 989cm-1, OOH group: 3550cm-1 |
| NMR Spectra | 1H-NMR Chan_HW et al. Frankel_EN et al., 1H-NMR( after methanolyzation and reduction) Gardner_HW et al. Neff_WE et al.: trans,cis isomer: C10-13 (5.42-6.48ppm), C14 (2.10-2.18ppm), C9(4.15ppm), J10-11= 15.4Hz(trans), J12-13= 10.8Hz (cis), trans, trans isomer: olefinic protons (5.41ppm), C14 (2.07ppm), C9 (4.20ppm) |
| Other Spectra | |
| Chromatograms | |