LBF18206HP01
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8001 |
| LipidMaps | LMFA01040004 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18206HP01 |
| 9-HPODE | |
|---|---|
| |
| Structural Information | |
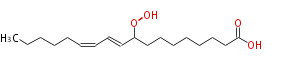
| 9-Hydroperoxy-10,12-octadecadienoic acid | |
| |
| Formula | C18H32O4 |
| Exact Mass | 312.23005951199997 |
| Average Mass | 312.44428 |
| SMILES | CCCCCC=CC=CC(OO)CCCCCCCC(O)=O |
| Physicochemical Information | |
| Auto oxidation of methyllinoleate Frankel_EN Frankel_EN Frankel_EN Frankel_EN Chan_HWS et al.. Oxidation of methyl linoleate by singlet oxygen Frankel_EN Frankel_EN Frankel_EN Frankel_EN . Oxidation of linoleic acid by lipoxygenase Mathuo_M Wakabayashi_T . Production mechanism (auto oxidation): bis-allylic hydrogen at C11. | |
| Pysiological damages are induced by these hydroperoxides which are incorporated into bodies or synthesized endogenously. Logani_MK et al. Sevanian_A et al. Fujimoto_K | |
| Spectral Information | |
| Mass Spectra | GC/EI-MS(after methanolysis, reduction and trimethylsilylation) Frankel_EN et al. KleimanRet al. Gardner_HW et al. Frankel_EN et al. HambergM: m/e= 382[M], 292[M-HOTMS], 311[M-(CH2)4CH3], 225[M-(CH2)7COOCH3] standard peak/ GC-EI-MS(after methylation, reduction and hydrogenation) Chan_HWS DolevAet al. Zimmerman_DC et al.: m/e= 187[CH(OH)(CH2)7COOCH3], 158[(CH2)7COOCH3+H], 155[C(OH)-(CH)7CO] |
| UV Spectra | Trans, cis isomer: λ max=236nm, ε =25900, trans, trans isomer: λ max=233nm, ε =28600 Chan_HW et al. Bolland_JL et al. Lundberg_WO et al. Lundberg_WO et al. Gardner_HW et al. Gardner_HW et al. |
| IR Spectra | Methyl ester: Chan_HW et al. Gardner_HW et al. Cannon_JA et al. Privett_OS et al. Sephton_HH et al. Graveland_A_ Privett_OS et al. Gardner_HW et al.: trans, cis isomer: 986 and 949cm-1, trans, trans isomer: 989cm-1, OOH group: 3550cm-1 |
| NMR Spectra | 1H-NMR Chan_HW et al. Frankel_EN et al., 1H-NMR( after methanolyzation and reduction) Gardner_HW et al. Neff_WE et al.: trans,cis isomer: C10-13 (5.42-6.48ppm), C14 (2.10-2.18ppm), C9(4.15ppm), J10-11= 15.4Hz(trans), J12-13= 10.8Hz (cis), trans, trans isomer: olefinic protons (5.41ppm), C14 (2.07ppm), C9 (4.20ppm) |
| Other Spectra | |
| Chromatograms | |








