LBF18109SC01: Difference between revisions
No edit summary |
mNo edit summary |
||
| (16 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 5: | Line 7: | ||
|LipidMaps=LMFA01030002 | |LipidMaps=LMFA01030002 | ||
|SysName=cis-9-Octadecenoic acid | |SysName=cis-9-Octadecenoic acid | ||
|Common Name= | |Common Name=&&Oleic acid&&9Z-Octadecenoic acid&& | ||
|Melting Point=12°C [labile] / 16°C [stable] | |Melting Point=12°C [labile] / 16°C [stable] | ||
|Boiling Point=234°C at 15 mmHg | |Boiling Point=234°C at 15 mmHg | ||
|Density= | |Density=d^{20}_4 0.898 | ||
| | |Refractive=1.45823 at 20°C | ||
|Solubility=soluble in acetone , alcohol, chloroform, ether and petroleum ether | |Solubility=soluble in acetone , alcohol, chloroform, ether and petroleum ether | ||
|Mass Spectra={{Image200|LBF18109SC01SP0001.gif}} (provided by Dr. Takeshi Kasama). | |Mass Spectra={{Image200|LBF18109SC01SP0001.gif}} (provided by Dr. Takeshi Kasama). | ||
|Chromatograms=Gas liquid chromatogram {{Image200|LBF18109SC01CH0001.gif}}{{Image200|LBF18109SC01CH0002.gif}} (provided by Dr. Akiko Horiuchi). | |Chromatograms=Gas liquid chromatogram {{Image200|LBF18109SC01CH0001.gif}}{{Image200|LBF18109SC01CH0002.gif}} (provided by Dr. Akiko Horiuchi). | ||
|Source=Generally considered to be predominant fatty acid in nature (few fats known to contain less than 10%; Separation from olive oil by double fraction via urea adduct<!--4003-->. Major constituent in plant oils e.g. olive oil (about 80%), almond oil (about 80%), mainly as glyceride. | |||
|Chemical Synthesis=Purest product (98%) obtained when the oil was saponified with 3% KOH solution; Synthesis<!--4008--> | |||
|Metabolism=In animals oleic acid is synthsized from stearoyl CoA by oxidative desaturation using O_2 , NADPH, cytochrome b_5 . Oleic acid is further metabolized to 20:9n-9 via reactions of desaturation and elongation. Essential fatty acid deficincy causes the accumulation of 20:9n-9. | |||
|Symbol=Ole / C18:1n-9 | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 08:53, 20 July 2016
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA0111 |
| LipidMaps | LMFA01030002 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18109SC01 |
| Oleic acid | |
|---|---|
| |
| Structural Information | |
| cis-9-Octadecenoic acid | |
| |
| Ole / C18:1n-9 | |
| Formula | C18H34O2 |
| Exact Mass | 282.255880332 |
| Average Mass | 282.46136 |
| SMILES | CCCCCCCCC=CCCCCCCCC(O)=O |
| Physicochemical Information | |
| 12°C [labile] / 16°C [stable] | |
| 234°C at 15 mmHg | |
| d20 4 0.898 | |
| 1.45823 at 20°C | |
| soluble in acetone , alcohol, chloroform, ether and petroleum ether | |
| Generally considered to be predominant fatty acid in nature (few fats known to contain less than 10%; Separation from olive oil by double fraction via urea adduct. Major constituent in plant oils e.g. olive oil (about 80%), almond oil (about 80%), mainly as glyceride. | |
| Purest product (98%) obtained when the oil was saponified with 3% KOH solution; Synthesis | |
| In animals oleic acid is synthsized from stearoyl CoA by oxidative desaturation using O_2 , NADPH, cytochrome b_5 . Oleic acid is further metabolized to 20:9n-9 via reactions of desaturation and elongation. Essential fatty acid deficincy causes the accumulation of 20:9n-9. | |
| Spectral Information | |
| Mass Spectra | 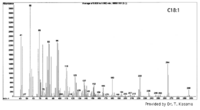 (provided by Dr. Takeshi Kasama). |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | Gas liquid chromatogram 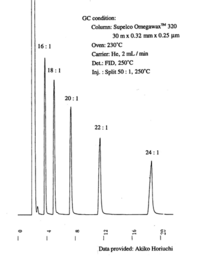 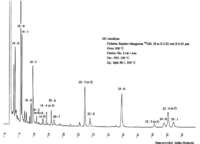 (provided by Dr. Akiko Horiuchi). |