LBF16000PG01: Difference between revisions
No edit summary |
No edit summary |
||
| (17 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=XPR1421 | |LipidBank=XPR1421 | ||
|LipidMaps=LMFA03010136 | |LipidMaps=LMFA03010136 | ||
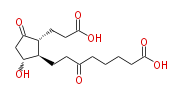
|SysName=8-[ | |SysName=8- [ 2R- (2-Carboxyethyl) -5R-hydroxy-3-oxocyclopentan- 1R-yl ] -6-oxooctanoic acid | ||
|Common Name=&&8-[2(R)-(2-Carboxyethyl)-5(R)-hydroxy-3-oxocyclopentan-1(R)-yl]-6-oxooctanoic acid&& | |Common Name=&&8- [ 2 (R) - (2-Carboxyethyl) -5 (R) -hydroxy-3-oxocyclopentan-1 (R) -yl ] -6-oxooctanoic acid&& | ||
|Solubility=ETHYL ACETATE [[Reference:Hamberg_M:Samuelsson_B:,J. Am. Chem. Soc.,1969,91,2177|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,J. Am. Chem. Soc.,1969,91,2177}}]] | |Solubility=ETHYL ACETATE [[Reference:Hamberg_M:Samuelsson_B:,J. Am. Chem. Soc.,1969,91,2177|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,J. Am. Chem. Soc.,1969,91,2177}}]] | ||
|Mass Spectra=DIMETHYL ESTER DI-O-METHYLOXIME TMS ETHER DERIVATIVE ; m/e 486, 455, 369, 365, 355, 286, 279, 268, 265, 196, 115 [[Reference:Hamberg_M:Samuelsson_B:,J. Am. Chem. Soc.,1969,91,2177|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,J. Am. Chem. Soc.,1969,91,2177}}]] | |Mass Spectra=DIMETHYL ESTER DI-O-METHYLOXIME TMS ETHER DERIVATIVE ; m/e 486, 455, 369, 365, 355, 286, 279, 268, 265, 196, 115 [[Reference:Hamberg_M:Samuelsson_B:,J. Am. Chem. Soc.,1969,91,2177|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,J. Am. Chem. Soc.,1969,91,2177}}]] | ||
|Source=When prostaglandin E2 or E1 was administered intravenously to man, 7 alpha -hydroxy-5,11-diketo-tetranor-prosta-1,16-dioic acid was found as a major urinary metabolite (PGE-MUM) [[Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1971,246,6713|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1971,246,6713}}]]. | |||
|Chemical Synthesis= | |||
|Metabolism= | |||
|Symbol=PGE-MUM | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 15:38, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1421 |
| LipidMaps | LMFA03010136 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF16000PG01 |
| 8- [ 2 (R) - (2-Carboxyethyl) -5 (R) -hydroxy-3-oxocyclopentan-1 (R) -yl ] -6-oxooctanoic acid | |
|---|---|
| |
| Structural Information | |
| 8- [ 2R- (2-Carboxyethyl) -5R-hydroxy-3-oxocyclopentan- 1R-yl ] -6-oxooctanoic acid | |
| |
| PGE-MUM | |
| Formula | C16H24O7 |
| Exact Mass | 328.152203122 |
| Average Mass | 328.35756000000003 |
| SMILES | OC(=O)CCCCC(=O)CC[C@@H]([C@H](O)1)[C@@H](CCC(O)=O)C(=O)C1 |
| Physicochemical Information | |
| ETHYL ACETATE HambergMet al. | |
| When prostaglandin E2 or E1 was administered intravenously to man, 7 alpha -hydroxy-5,11-diketo-tetranor-prosta-1,16-dioic acid was found as a major urinary metabolite (PGE-MUM) Hamberg_M et al.. | |
| Spectral Information | |
| Mass Spectra | DIMETHYL ESTER DI-O-METHYLOXIME TMS ETHER DERIVATIVE ; m/e 486, 455, 369, 365, 355, 286, 279, 268, 265, 196, 115 HambergMet al. |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|