LBF18206SC05: Difference between revisions
No edit summary |
No edit summary |
||
| (8 intermediate revisions by 2 users not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=DFA0159 | |LipidBank=DFA0159 | ||
|LipidMaps=LMFA01030120 | |LipidMaps=LMFA01030120 | ||
|SysName=cis-9, cis-12-Octadecadienoic acid | |SysName=(cis-9,cis-12) -Octadecadienoic acid | ||
|Common Name=&& | |Common Name=&&(9Z,12Z) -Octadecadienoic acid&&Linoleic acid&& | ||
|Melting Point=-5°C | |Melting Point=-5°C | ||
|Boiling Point=229°C to 230°C at 16mmHg | |Boiling Point=229°C to 230°C at 16mmHg | ||
|Density= | |Density=d^{20}_4 0.9031 | ||
|Refractive=1.4711 at 20°C | |Refractive=1.4711 at 20°C | ||
|Solubility=soluble in acetone, alcohol, ether and petroleum ether.[[Reference:Matthews_NL:Brode_WR:Brown_JB:,J. Am. Chem. Soc.,1941,63,1064|{{RelationTable/GetFirstAuthor|Reference:Matthews_NL:Brode_WR:Brown_JB:,J. Am. Chem. Soc.,1941,63,1064}}]][[Reference:Nicolaides_N:Laves_F:,J. Am. Chem. Soc.,1958,80,5752|{{RelationTable/GetFirstAuthor|Reference:Nicolaides_N:Laves_F:,J. Am. Chem. Soc.,1958,80,5752}}]] | |Solubility=soluble in acetone, alcohol, ether and petroleum ether.<!--0193-->[[Reference:Matthews_NL:Brode_WR:Brown_JB:,J. Am. Chem. Soc.,1941,63,1064|{{RelationTable/GetFirstAuthor|Reference:Matthews_NL:Brode_WR:Brown_JB:,J. Am. Chem. Soc.,1941,63,1064}}]][[Reference:Nicolaides_N:Laves_F:,J. Am. Chem. Soc.,1958,80,5752|{{RelationTable/GetFirstAuthor|Reference:Nicolaides_N:Laves_F:,J. Am. Chem. Soc.,1958,80,5752}}]]<!--0409--><!--0410--> | ||
|Chromatograms=Gas liquid chromatogram {{Image200|LBF18206SC05CH0001.gif}} (provided by Dr. Akiko Horiuchi). | |Chromatograms=Gas liquid chromatogram {{Image200|LBF18206SC05CH0001.gif}} (provided by Dr. Akiko Horiuchi). | ||
|Source=Constituent of the essential fatty acids (vitamin F) and of various microorganisms; oils of cottonseed, soybean, peanut, corn, sunflower and poppy seed. | |Source=Constituent of the essential fatty acids (vitamin F) and of various microorganisms; oils of cottonseed, soybean, peanut, corn, sunflower and poppy seed. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism=Linoleic acid (18:2n-6) is synthesized from oleic acid (18:1n-9) by desaturation of | |Metabolism=Linoleic acid (18:2n-6) is synthesized from oleic acid (18:1n-9) by desaturation of Delta 12-desaturase, and alpha -linolenic acid (18:3n-3) is formed from linoleic acid by desaturation reaction of Delta 15-desaturase. Since both Delta 12- and 15-desaturases are present in plant cells but not in animal cells, linoleic and alpha -linolenic acid are not biosynthesized in animal cells in vivo. When ingested by animals, linoleic acid is desaturated, elongated to form gamma -linolenic acid (18:3n-6), dihomo- gamma -linolenic acid (20:3n-6), arachidonic acid (20:4n-6) and adrenic acid (22:4n-6). Docosapentaenoic acid (22:5n-6) is synthesized from adrenic acid in significant amounts only under conditions of prolonged n-3 fatty acid deficiency., No interconversion between the n-6 and n-3 series in mammals. Nutritionally, it is important to note that different foods contain different proportions of n-6/n-3 and therefore the n-6/n-3 ratio in tissue lipids change significantly depending on the choice of foods.[[Reference:Okuyama_H:Kobayashi_T:Watanabe_S:,Prog. Lipid Res.,1996,35,409|{{RelationTable/GetFirstAuthor|Reference:Okuyama_H:Kobayashi_T:Watanabe_S:,Prog. Lipid Res.,1996,35,409}}]] Although plants synthesize and store linoleic acid and alpha -linolenic acid as well as saturated and monounsaturated fatty acids in grains, the proportions of these fatty acids in different vegetable oils differ greatly. Safflower and sunflower oil contain high levels of linoleate, while perilla and linseed oil are rich in alpha -linolenic acid. | ||
|Symbol=LA / C18:2n-6 / C18:2 | |Symbol=LA / C18:2n-6 / C18:2 omega 6 | ||
|Biological Activity=There are two groups of essential fatty acids for mammals, the n-6 (or | |Biological Activity=There are two groups of essential fatty acids for mammals, the n-6 (or omega 6) and the n-3 (or omega 3). Linoleic acid (LA) is one of the n-6 essential fatty. Animals deficient in LA show the growth retardation, skin lesions, reproductive failure, fatty liver and polydipsia <!--2009-->[[Reference:Paulsrud_JR:Pensler_L:Whitten_CF:Stewart_S:Holman:RT:,Am. J. Clin. Nutr.,1972,25,897|{{RelationTable/GetFirstAuthor|Reference:Paulsrud_JR:Pensler_L:Whitten_CF:Stewart_S:Holman:RT:,Am. J. Clin. Nutr.,1972,25,897}}]]. Dietary supplementation of either linoleate, gamma -linoleate or arachidonate prevents such symptoms completely. Its physiological functions appear to be mediated mainly through the hormone-like eicosanoids (prostaglandins, leukotriens and thromboxanes et al.) derived from arachidonic acid which is biosynthesized from LA. LA is also shown to be involved in control of the water impermeability of the skin and regulation of cholesterol synthesis and transport. | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 19:28, 1 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA0159 |
| LipidMaps | LMFA01030120 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18206SC05 |
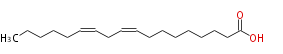
| (9Z,12Z) -Octadecadienoic acid | |
|---|---|
| |
| Structural Information | |
| (cis-9,cis-12) -Octadecadienoic acid | |
| |
| LA / C18:2n-6 / C18:2 omega 6 | |
| Formula | C18H32O2 |
| Exact Mass | 280.240230268 |
| Average Mass | 280.44548000000003 |
| SMILES | CCCCCC=CCC=CCCCCCCCC(O)=O |
| Physicochemical Information | |
| -5°C | |
| 229°C to 230°C at 16mmHg | |
| d20 4 0.9031 | |
| 1.4711 at 20°C | |
| soluble in acetone, alcohol, ether and petroleum ether. Matthews_NL et al. NicolaidesNet al. | |
| Constituent of the essential fatty acids (vitamin F) and of various microorganisms; oils of cottonseed, soybean, peanut, corn, sunflower and poppy seed. | |
| Linoleic acid (18:2n-6) is synthesized from oleic acid (18:1n-9) by desaturation of Delta 12-desaturase, and alpha -linolenic acid (18:3n-3) is formed from linoleic acid by desaturation reaction of Delta 15-desaturase. Since both Delta 12- and 15-desaturases are present in plant cells but not in animal cells, linoleic and alpha -linolenic acid are not biosynthesized in animal cells in vivo. When ingested by animals, linoleic acid is desaturated, elongated to form gamma -linolenic acid (18:3n-6), dihomo- gamma -linolenic acid (20:3n-6), arachidonic acid (20:4n-6) and adrenic acid (22:4n-6). Docosapentaenoic acid (22:5n-6) is synthesized from adrenic acid in significant amounts only under conditions of prolonged n-3 fatty acid deficiency., No interconversion between the n-6 and n-3 series in mammals. Nutritionally, it is important to note that different foods contain different proportions of n-6/n-3 and therefore the n-6/n-3 ratio in tissue lipids change significantly depending on the choice of foods. Okuyama_H et al. Although plants synthesize and store linoleic acid and alpha -linolenic acid as well as saturated and monounsaturated fatty acids in grains, the proportions of these fatty acids in different vegetable oils differ greatly. Safflower and sunflower oil contain high levels of linoleate, while perilla and linseed oil are rich in alpha -linolenic acid. | |
| There are two groups of essential fatty acids for mammals, the n-6 (or omega 6) and the n-3 (or omega 3). Linoleic acid (LA) is one of the n-6 essential fatty. Animals deficient in LA show the growth retardation, skin lesions, reproductive failure, fatty liver and polydipsia Paulsrud_JR et al.. Dietary supplementation of either linoleate, gamma -linoleate or arachidonate prevents such symptoms completely. Its physiological functions appear to be mediated mainly through the hormone-like eicosanoids (prostaglandins, leukotriens and thromboxanes et al.) derived from arachidonic acid which is biosynthesized from LA. LA is also shown to be involved in control of the water impermeability of the skin and regulation of cholesterol synthesis and transport. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | Gas liquid chromatogram  (provided by Dr. Akiko Horiuchi). |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|