LBF20207HP02: Difference between revisions
New page: {{Lipid/Header}} {{Hierarchy|{{PAGENAME}}}} {{Metabolite |LipidBank=DFA8091 |LipidMaps=- |SysName=7- [ 3,5-Epidioxy-2- (3-hydroperoxy-1-octenyl) cycropentyl ] -5-heptenoic acid |Common N... |
No edit summary |
||
| Line 6: | Line 6: | ||
|LipidBank=DFA8091 | |LipidBank=DFA8091 | ||
|LipidMaps=- | |LipidMaps=- | ||
|SysName=7- [ 3,5-Epidioxy-2- (3-hydroperoxy-1-octenyl) cycropentyl ] -5-heptenoic acid | |SysName=7- [3,5-Epidioxy-2- (3-hydroperoxy-1-octenyl) cycropentyl] -5-heptenoic acid | ||
|Common Name=&&7- [ 3,5-Epidioxy-2- (3-hydroperoxy-1-octenyl) cycropentyl ] -5-heptenoic acid&& | |Common Name=&&7- [3,5-Epidioxy-2- (3-hydroperoxy-1-octenyl) cycropentyl] -5-heptenoic acid&& | ||
|Mass Spectra=GC-EI-MS(Me-ester; after reduction and TMS)[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595}}]]: m/e=569[M-CH3]; 513[M-(CH2)4CH3]; 494[M-HOTMS]; 404[M-2xHOTMS]; 378[494-SMTO=CHCH2]; 367[M-SMTOCH=CHCH=OTMS]; 191[SMTO=CHOTMS]; 173[SMTO=CH(CH2)4CH3]; GC-EI-MS(Me-ester; after reduction, hydrogenation and TMS) | |Mass Spectra=GC-EI-MS(Me-ester; after reduction and TMS)[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595}}]]: m/e=569[M-CH3]; 513[M-(CH2)4CH3]; 494[M-HOTMS]; 404[M-2xHOTMS]; 378[494-SMTO=CHCH2]; 367[M-SMTOCH=CHCH=OTMS]; 191[SMTO=CHOTMS]; 173[SMTO=CH(CH2)4CH3]; GC-EI-MS(Me-ester; after reduction, hydrogenation and TMS) | ||
|Source=Oxidation of arachidonic acid in the presence of hemoglobin, mioglobin[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595}}]]or Fe(III)- ascorbic acid[[Reference:Yamagata_S:Murakami_H:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1983,47,2791|{{RelationTable/GetFirstAuthor|Reference:Yamagata_S:Murakami_H:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1983,47,2791}}]]. It is produced from arachidonic acid by cyclooxygenase in vivo(PGG2)<!--8100--><!--8103--><!--8104-->. | |Source=Oxidation of arachidonic acid in the presence of hemoglobin, mioglobin[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595}}]]or Fe(III)- ascorbic acid[[Reference:Yamagata_S:Murakami_H:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1983,47,2791|{{RelationTable/GetFirstAuthor|Reference:Yamagata_S:Murakami_H:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1983,47,2791}}]]. It is produced from arachidonic acid by cyclooxygenase in vivo(PGG2)<!--8100--><!--8103--><!--8104-->. | ||
Latest revision as of 18:17, 22 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8091 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207HP02 |
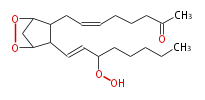
| 7- [3,5-Epidioxy-2- (3-hydroperoxy-1-octenyl) cycropentyl] -5-heptenoic acid | |
|---|---|
| |
| Structural Information | |
| 7- [3,5-Epidioxy-2- (3-hydroperoxy-1-octenyl) cycropentyl] -5-heptenoic acid | |
| |
| Formula | C20H32O6 |
| Exact Mass | 368.219888756 |
| Average Mass | 368.46448 |
| SMILES | C(CCC(OO)C=CC(C21)C(CC=CCCCC(O)=O)C(OO2)C1)CC |
| Physicochemical Information | |
| Oxidation of arachidonic acid in the presence of hemoglobin, mioglobin Terao_J et al.or Fe(III)- ascorbic acid Yamagata_S et al.. It is produced from arachidonic acid by cyclooxygenase in vivo(PGG2). | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(Me-ester; after reduction and TMS) TeraoJet al.: m/e=569[M-CH3]; 513[M-(CH2)4CH3]; 494[M-HOTMS]; 404[M-2xHOTMS]; 378[494-SMTO=CHCH2]; 367[M-SMTOCH=CHCH=OTMS]; 191[SMTO=CHOTMS]; 173[SMTO=CH(CH2)4CH3]; GC-EI-MS(Me-ester; after reduction, hydrogenation and TMS) |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|