LBF20303PG03: Difference between revisions
No edit summary |
No edit summary |
||
| (10 intermediate revisions by 2 users not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=XPR1402 | |LipidBank=XPR1402 | ||
|LipidMaps=LMFA03010135 | |LipidMaps=LMFA03010135 | ||
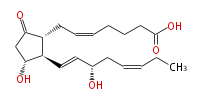
|SysName=7- [ | |SysName=7- [3R-Hydroxy-2R- (3S-hydroxyocta- (trans-1,cis-5) -dienyl) -5-oxocyclopentan-1R-yl ] -cis-5-heptenoic acid | ||
|Common Name=&&Prostaglandin E_3&&(5Z,8R,11R,12R,13E,15S,17Z) -11,15-Dihydroxy-9-oxo-5,13,17-prostatrienoic acid&&7- [ 3 (R) -Hydroxy-2 (R) - (3 (S) -hydroxyocta-1 (E) ,5 (Z) -dienyl) -5-oxocyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid&& | |||
|Melting Point=84.5-85.5°C | |Melting Point=84.5-85.5°C [[Reference:Okamoto K:Kobayashi_Y:Sato_F:,Tetrahedron Lett.,1989,30,4379|{{RelationTable/GetFirstAuthor|Reference:Okamoto K:Kobayashi_Y:Sato_F:,Tetrahedron Lett.,1989,30,4379}}]] | ||
| | |Optical=[ alpha ]^{24}_D =-48.9°(C=1.2, TETRAHYDROFURAN) [[Reference:Corey_EJ:Shirahama_H:Yamamoto_H:Terashima_S:Venkateswarlu_A:Schaaf_TK:,J. Am. Chem. Soc.,1971,93,1490|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Shirahama_H:Yamamoto_H:Terashima_S:Venkateswarlu_A:Schaaf_TK:,J. Am. Chem. Soc.,1971,93,1490}}]] | ||
|Solubility=TETRAHYDROFURAN [[Reference:Corey_EJ:Shirahama_H:Yamamoto_H:Terashima_S:Venkateswarlu_A:Schaaf_TK:,J. Am. Chem. Soc.,1971,93,1490|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Shirahama_H:Yamamoto_H:Terashima_S:Venkateswarlu_A:Schaaf_TK:,J. Am. Chem. Soc.,1971,93,1490}}]] | |Solubility=TETRAHYDROFURAN [[Reference:Corey_EJ:Shirahama_H:Yamamoto_H:Terashima_S:Venkateswarlu_A:Schaaf_TK:,J. Am. Chem. Soc.,1971,93,1490|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Shirahama_H:Yamamoto_H:Terashima_S:Venkateswarlu_A:Schaaf_TK:,J. Am. Chem. Soc.,1971,93,1490}}]] | ||
|Mass Spectra=METHYL ESTER ; m/e 346(M-18), 328(M-18x2), 315, 297, 277, 259, 188 [[Reference:Bergstroem_S:Dressler_F:Ryhage_R:Samuelsson_B:Sjoevall_J:,Ark. Kemi.,1962,19,563|{{RelationTable/GetFirstAuthor|Reference:Bergstroem_S:Dressler_F:Ryhage_R:Samuelsson_B:Sjoevall_J:,Ark. Kemi.,1962,19,563}}]] | |Mass Spectra=METHYL ESTER ; m/e 346(M-18), 328(M-18x2), 315, 297, 277, 259, 188 [[Reference:Bergstroem_S:Dressler_F:Ryhage_R:Samuelsson_B:Sjoevall_J:,Ark. Kemi.,1962,19,563|{{RelationTable/GetFirstAuthor|Reference:Bergstroem_S:Dressler_F:Ryhage_R:Samuelsson_B:Sjoevall_J:,Ark. Kemi.,1962,19,563}}]] | ||
|NMR Spectra=METHYL ESTER ; | |NMR Spectra=METHYL ESTER ; ^1 H-NMR(CDCl_3 ) : delta 5.8-5.5(m, 2H,13, 14-CH), 5.5-5.2(m, 4H, 5,6,17,18-CH), 4.4-3.8(m, 2H, 11,15-CH), 0.95(t, 3H, 20-CH) [[Reference:Samuelsson_B:,J. Am. Chem. Soc.,1963,85,1878|{{RelationTable/GetFirstAuthor|Reference:Samuelsson_B:,J. Am. Chem. Soc.,1963,85,1878}}]] | ||
|Source=Prostaglandin E3 is contained in human seminal plasma in an amount of 5.5 micrograms/ml [[Reference:Bergstrom_S:,Science,1967,157,382|{{RelationTable/GetFirstAuthor|Reference:Bergstrom_S:,Science,1967,157,382}}]] | |Source=Prostaglandin E3 is contained in human seminal plasma in an amount of 5.5 micrograms/ml [[Reference:Bergstrom_S:,Science,1967,157,382|{{RelationTable/GetFirstAuthor|Reference:Bergstrom_S:,Science,1967,157,382}}]], and also found in ovine seminal vesicle and plasma [[Reference:Speroff_L:Ramwell_PW:,Am. J. Obstet. Gynecol.,1970,107,1111|{{RelationTable/GetFirstAuthor|Reference:Speroff_L:Ramwell_PW:,Am. J. Obstet. Gynecol.,1970,107,1111}}]]. | ||
|Chemical Synthesis=[[Reference:Corey_EJ:Shirahama_H:Yamamoto_H:Terashima_S:Venkateswarlu_A:Schaaf_TK:,J. Am. Chem. Soc.,1971,93,1490|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Shirahama_H:Yamamoto_H:Terashima_S:Venkateswarlu_A:Schaaf_TK:,J. Am. Chem. Soc.,1971,93,1490}}]] | |Chemical Synthesis=[[Reference:Corey_EJ:Shirahama_H:Yamamoto_H:Terashima_S:Venkateswarlu_A:Schaaf_TK:,J. Am. Chem. Soc.,1971,93,1490|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Shirahama_H:Yamamoto_H:Terashima_S:Venkateswarlu_A:Schaaf_TK:,J. Am. Chem. Soc.,1971,93,1490}}]] {{Image200|LBF20303PG03FT0001.gif}} | ||
|Metabolism= | |Metabolism= | ||
|Symbol=PGE3 | |||
|Biological Activity=As presented in Table 1 of reference [[Reference:Bergstrom_S:Carlson_LA:Weeks_JR:,Pharmacol. Rev.,1968,20,1|{{RelationTable/GetFirstAuthor|Reference:Bergstrom_S:Carlson_LA:Weeks_JR:,Pharmacol. Rev.,1968,20,1}}]], prostaglandin E3 is 1/10-1/2 as active as prostaglandin E2. | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 17:55, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1402 |
| LipidMaps | LMFA03010135 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20303PG03 |
| Prostaglandin E3 | |
|---|---|
| |
| Structural Information | |
| 7- [3R-Hydroxy-2R- (3S-hydroxyocta- (trans-1,cis-5) -dienyl) -5-oxocyclopentan-1R-yl ] -cis-5-heptenoic acid | |
| |
| PGE3 | |
| Formula | C20H30O5 |
| Exact Mass | 350.20932407 |
| Average Mass | 350.4492 |
| SMILES | C(=CC[C@@H](O)C=C[C@H]([C@H]1CC=CCCCC(O)=O)[C@@H](CC1=O)O)CC |
| Physicochemical Information | |
| 84.5-85.5°C Okamoto K et al. | |
| [ α ]24 D =-48.9°(C=1.2, TETRAHYDROFURAN) Corey_EJ et al. | |
| TETRAHYDROFURAN Corey_EJ et al. | |
| Prostaglandin E3 is contained in human seminal plasma in an amount of 5.5 micrograms/ml Bergstrom_S , and also found in ovine seminal vesicle and plasma Speroff_L et al.. | |
Corey_EJ et al.  | |
| As presented in Table 1 of reference Bergstrom_S et al., prostaglandin E3 is 1/10-1/2 as active as prostaglandin E2. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER ; m/e 346(M-18), 328(M-18x2), 315, 297, 277, 259, 188 BergstroemSet al. |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | METHYL ESTER ; 1H-NMR(CDCl3) : δ 5.8-5.5(m, 2H,13, 14-CH), 5.5-5.2(m, 4H, 5,6,17,18-CH), 4.4-3.8(m, 2H, 11,15-CH), 0.95(t, 3H, 20-CH) SamuelssonB |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|