LBF20307PG01: Difference between revisions
No edit summary |
No edit summary |
||
| (15 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=XPR1001 | |LipidBank=XPR1001 | ||
|LipidMaps=LMFA03010035 | |LipidMaps=LMFA03010035 | ||
|SysName= | |SysName=9-Oxo-15S-hydroxy- (cis-5,cis-10,trans-13) -prostatrienoic acid | ||
|Common Name=&& | |Common Name=&&Prostaglandin A_2&&7- [2R- (3S-Hydroxy-1-(E)-octenyl) -5-oxo-3-cyclopenten-1R-yl] -5-(Z) -heptenoic acid&&9-Oxo-15S-hydroxy- (5Z,10Z,13E) -prostatrienoic acid&& | ||
| | |Optical=[ alpha ]X^{20}_D =+140°(C=1.15, CHLOROFORM) [[Reference:Corey_EJ:Moinet_G:,J. Am. Chem. Soc.,1973,95,6331|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Moinet_G:,J. Am. Chem. Soc.,1973,95,6331}}]] | ||
|Solubility=ETHANOL, CHLOROFORM, METHANOL, DIETHYLETHER [[Reference: | |Solubility=ETHANOL, CHLOROFORM, METHANOL, DIETHYLETHER [[Reference:Pike_JE:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_JE:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]] | ||
|Mass Spectra=m/e 334(M | |Mass Spectra=m/e 334(M^+ ), 316, 190 [[Reference:Pike_JE:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_JE:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]] | ||
|UV Spectra=EtOH: 217 nm ( | |UV Spectra=EtOH: 217 nm ( epsilon 9900) [[Reference:Pike_JE:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_JE:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]] | ||
|IR Spectra=NUJOL : | |IR Spectra=NUJOL : nu 3400, 1705, 1580, 1255, 1115, 1070, 1015 cm^{-1} [[Reference:Pike_JE:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_JE:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]] | ||
|NMR Spectra= | |NMR Spectra=^1 H-NMR(CDCl_3 ) : delta 7.57(dd, 1H, 10-CH), 6.2(dd, 1H, 11-CH), 5.6(m, 2H, 13,14-CH), 5.4 (m, 2H, 5,6-CH), 4.12(1H, m, 12-CH), 3.23(m, 1H), 0.89(t, 3H, 0-CH3) [[Reference:Schneider_WP:Bundy_GL:Lincoln_FH:Daniels_EG:Pike_JE:,J. Am. Chem. Soc.,1977,99,1222|{{RelationTable/GetFirstAuthor|Reference:Schneider_WP:Bundy_GL:Lincoln_FH:Daniels_EG:Pike_JE:,J. Am. Chem. Soc.,1977,99,1222}}]]. METHYL ESTER : ^{13}C-NMR(CDCl3) 210.3, 174.0, 165.0, 135.2, 133.3, 131.0, 130.2, 126.7, 72.4, 52.1, 51.5, 49.6, 37.3, 33.5, 31.8, 27.4, 26.7, 25.1, 24.8, 22.6, 14.0 [[Reference:Cooper_GF:Fried_J:,Proc. Natl. Acad. Sci. U. S. A.,1973,70,1579|{{RelationTable/GetFirstAuthor|Reference:Cooper_GF:Fried_J:,Proc. Natl. Acad. Sci. U. S. A.,1973,70,1579}}]] | ||
|Source=Prostaglandin A2 was found in human semen in an amount of about 50 micrograms per ml as measured in combination with prostaglandins A1, B1 and B2 [[Reference:Bergstrom_S:,Science,1967,157,382|{{RelationTable/GetFirstAuthor|Reference:Bergstrom_S:,Science,1967,157,382}}]]. | |||
|Chemical Synthesis=[[Reference:Corey_EJ:Moinet_G:,J. Am. Chem. Soc.,1973,95,6331|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Moinet_G:,J. Am. Chem. Soc.,1973,95,6331}}]] {{Image200|LBF20307PG01FT0001.gif}} | |||
|Metabolism=Prostaglandin A2 is produced by non-enzymatic degradation of prostaglandin E2 [[Reference:Pike_JE:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_JE:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]], and human serum has an enzymatic activity to convert prostaglandin E2 to A2 [[Reference:Polet_H:Levine_L:,J. Biol. Chem.,1975,250,351|{{RelationTable/GetFirstAuthor|Reference:Polet_H:Levine_L:,J. Biol. Chem.,1975,250,351}}]]. | |||
|Symbol=PGA2 | |||
|Biological Activity=Antitumor activity of prostaglandin A2 is known [[Reference:Fukushima_M:,Eicosanoids,1990,3,189|{{RelationTable/GetFirstAuthor|Reference:Fukushima_M:,Eicosanoids,1990,3,189}}]], and prostaglandin A2 rduces blood pressure [[Reference:Bergstrom_S:Carlson_LA:Weeks_JR:,Pharmacol. Rev.,1968,20,1|{{RelationTable/GetFirstAuthor|Reference:Bergstrom_S:Carlson_LA:Weeks_JR:,Pharmacol. Rev.,1968,20,1}}]]. | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 20:53, 2 November 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1001 |
| LipidMaps | LMFA03010035 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20307PG01 |
| Prostaglandin A2 | |
|---|---|

| |
| Structural Information | |
| 9-Oxo-15S-hydroxy- (cis-5,cis-10,trans-13) -prostatrienoic acid | |
| |
| PGA2 | |
| Formula | C20H30O4 |
| Exact Mass | 334.21440944799997 |
| Average Mass | 334.4498 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]1CC=CCCCC(O)=O)C=CC(=O)1)CC |
| Physicochemical Information | |
| [ α ]X20 D =+140°(C=1.15, CHLOROFORM) Corey_EJ et al. | |
| ETHANOL, CHLOROFORM, METHANOL, DIETHYLETHER Pike_JE et al. | |
| Prostaglandin A2 was found in human semen in an amount of about 50 micrograms per ml as measured in combination with prostaglandins A1, B1 and B2 Bergstrom_S . | |
Corey_EJ et al. 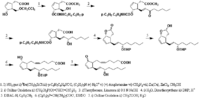 | |
| Prostaglandin A2 is produced by non-enzymatic degradation of prostaglandin E2 Pike_JE et al., and human serum has an enzymatic activity to convert prostaglandin E2 to A2 Polet_H et al.. | |
| Antitumor activity of prostaglandin A2 is known Fukushima_M , and prostaglandin A2 rduces blood pressure Bergstrom_S et al.. | |
| Spectral Information | |
| Mass Spectra | m/e 334(M+), 316, 190 Pike_JE et al. |
| UV Spectra | EtOH: 217 nm ( ε 9900) Pike_JE et al. |
| IR Spectra | NUJOL : ν 3400, 1705, 1580, 1255, 1115, 1070, 1015 cm-1 Pike_JE et al. |
| NMR Spectra | 1H-NMR(CDCl3) : δ 7.57(dd, 1H, 10-CH), 6.2(dd, 1H, 11-CH), 5.6(m, 2H, 13,14-CH), 5.4 (m, 2H, 5,6-CH), 4.12(1H, m, 12-CH), 3.23(m, 1H), 0.89(t, 3H, 0-CH3) Schneider_WP et al.. METHYL ESTER : 13C-NMR(CDCl3) 210.3, 174.0, 165.0, 135.2, 133.3, 131.0, 130.2, 126.7, 72.4, 52.1, 51.5, 49.6, 37.3, 33.5, 31.8, 27.4, 26.7, 25.1, 24.8, 22.6, 14.0 Cooper_GF et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|