LBF20406AM19: Difference between revisions
No edit summary |
No edit summary |
||
| (9 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=XPR7035 | |LipidBank=XPR7035 | ||
|LipidMaps=LMFA08020021 | |LipidMaps=LMFA08020021 | ||
|SysName=N- (2- | |SysName=N- (2-Methoxyethyl) - (cis-5,cis-8,cis-11,cis-14) -eicosatetraenoylamine | ||
|Common Name=&&N- (2- | |Common Name=&&N- (2-Methoxyethyl) -arachidonoylamide&&N- (2-Methoxyethyl) - (5Z,8Z,11Z,14Z) -eicosatetraenoylamine&& | ||
|Melting Point=colorless oil [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |Melting Point=colorless oil [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | ||
|NMR Spectra= | |NMR Spectra=^1 H NMR (CDCl3) delta 5.80 (br s lH), 5.30-5.42 (m, 8H), 3.45 (d, J=2.1Hz, 4H), 3.35 (s, 3H), 2.75-2.85 (m, 6H), 2.00-2.20 (m, 6H), 1.62-1.80 (m, 2H), 1.20-1.40 (m, 6H), 0.89 (t, J=6.8Hz, 3H) [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | ||
|Source= | |Source= | ||
|Chemical Synthesis=This compound was synthesized from arachidonoylchloride and methoxyethylamine. Yield 4l%. [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |Chemical Synthesis=This compound was synthesized from arachidonoylchloride and methoxyethylamine. Yield 4l%. [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | ||
|Metabolism= | |Metabolism= | ||
|Biological Activity=Binding to the rat brain cannabinoid receptor (CBl), Ki(nM)= 85.2±3.8 [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 13:43, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR7035 |
| LipidMaps | LMFA08020021 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406AM19 |
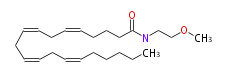
| N- (2-Methoxyethyl) -arachidonoylamide | |
|---|---|
| |
| Structural Information | |
| N- (2-Methoxyethyl) - (cis-5,cis-8,cis-11,cis-14) -eicosatetraenoylamine | |
| |
| Formula | C23H39NO2 |
| Exact Mass | 361.298079497 |
| Average Mass | 361.5613 |
| SMILES | O(C)CCNC(=O)CCCC=CCC=CCC=CCC=CCCCCC |
| Physicochemical Information | |
| colorless oil Sheskin_T et al. | |
| This compound was synthesized from arachidonoylchloride and methoxyethylamine. Yield 4l%. Sheskin_T et al. | |
| Binding to the rat brain cannabinoid receptor (CBl), Ki(nM)= 85.2±3.8 Sheskin_T et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 1H NMR (CDCl3) δ 5.80 (br s lH), 5.30-5.42 (m, 8H), 3.45 (d, J=2.1Hz, 4H), 3.35 (s, 3H), 2.75-2.85 (m, 6H), 2.00-2.20 (m, 6H), 1.62-1.80 (m, 2H), 1.20-1.40 (m, 6H), 0.89 (t, J=6.8Hz, 3H) SheskinTet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|