LBF20406CV10: Difference between revisions
New page: {{Lipid/Header}} {{Hierarchy|{{PAGENAME}}}} {{Metabolite |LipidBank=XPR8006 |LipidMaps=LMFA03120006 |SysName=Methyl-4R- (5-trans,7-trans) -4-acetoxy-7- [ 2S-acetoxy-2- [cis-7-acetoxy-2-o... |
(No difference)
|
Revision as of 18:05, 16 September 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR8006 |
| LipidMaps | LMFA03120006 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406CV10 |
| 20-Acetoxyclavulone II | |
|---|---|
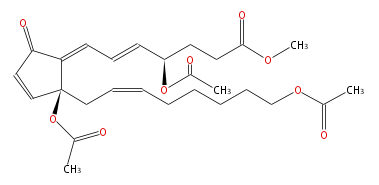
| |
| Structural Information | |
| Methyl-4R- (5-trans,7-trans) -4-acetoxy-7- [ 2S-acetoxy-2- [cis-7-acetoxy-2-octenyl] -5-oxo-3-cyclopentenylidene ] -5-heptenoic acid | |
| |
| Formula | C27H36O9 |
| Exact Mass | 504.23593274999996 |
| Average Mass | 504.56934 |
| SMILES | C(C[C@](OC(C)=O)(C=1)C(=CC=C[C@@H](CCC(=O)OC)OC(C)=O)C(=O)C1)=CCCCCCOC(C)=O |
| Physicochemical Information | |
| [ α ]D +3.7°(C 0.54, CHCl3) IguchiKet al. | |
| 20-Acetoxyclavulones are soluble in MeOH, EtOH, CHCl3, or hexane. | |
| 20-Acetoxyclavulones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard. Iguchi_K et al. | |
| 20-Acetoxyclavulones showed a significant anti-inflammatory effect at 30 mu g/ml by the fertile egg test. Kikuchi_H et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max(EtOH) 230 nm( ε 14200),292 nm( ε 18700) IguchiKet al. |
| IR Spectra | ν max(film)1730,1700,1640,and 1235cm-1 IguchiKet al. |
| NMR Spectra | 1H-NMR(270MHz,CDCl3) δ ppm2.05(3H,s),2.07(3H,s),2.08(3H,s),2.38(2H,t,J=7.3Hz),2.69(1H,dd,J=7.6,16Hz),2.87(1H,dd,J=7.3,16Hz),3.68(3H,s),4.04(2H,t,J=6.9Hz),5.20(1H,m),5.41(1H,q,J=7Hz),5.51(1H,dt,J=11,7.3Hz),6.03(1H,dd,J=7,14.8Hz),6.41(1H,d,J=6.9Hz),6.74(1H,dd,J=12.2,14.8Hz),6.86(1H,d,J=12.2Hz),7.47(1H,d,J=5.9Hz). IguchiKet al.13C-NMR(67.8MHz,CDCl3) δ ppm21.0(q,2C),21.2(q),25.6(t),27.3(t),28.5(t),29.2(t,2C),29.5(t),36.0(t),51.8(q),64.4(t),72.8(d),85.0(s),121.5(d),126.8(d),129.3(d),134.5(d),135.0(d),136.8(s),141.3(d),158.1(d),169.5(s),169.9(s),171.2(s),172.9(s),193.3(s). IguchiKet al. |
| Other Spectra | CD λ ext(EtOH)( Δ ε )nm 247(+2.6),295(-1.0). KikuchiHet al. IguchiKet al. |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|