LBF20407HO07: Difference between revisions
No edit summary |
No edit summary |
||
| (18 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=XPR6103 | |LipidBank=XPR6103 | ||
|LipidMaps=- | |LipidMaps=- | ||
|SysName=15 (S) -Hydroxy-5,8,11,13- (Z,Z,Z,E) -eicosatetraenoic acid | |SysName=15S-Hydroxy- (cis-5,cis-8,cis-11,trans-13) -eicosatetraenoic acid | ||
|Solubility=DIETHYL ETHER | |Common Name=&&15S-Hydroxy- (5Z,8Z,11Z,13E) -eicosatetraenoic acid&&15(S) -Hydroxy-5,8,11,13- (Z,Z,Z,E) -eicosatetraenoic acid&& | ||
|Mass Spectra=METHYL ESTER TMS ETHER ; m/e 406(M | |Solubility=DIETHYL ETHER [[Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115|{{RelationTable/GetFirstAuthor|Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115}}]] | ||
|UV Spectra=METHYL ESTER ; | |Mass Spectra=METHYL ESTER TMS ETHER ; m/e 406(M^+ ), 391, 335, 316, 305, 225, 173 [[Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115|{{RelationTable/GetFirstAuthor|Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115}}]] | ||
|NMR Spectra=METHYL ESTER ; | |UV Spectra=METHYL ESTER ; lambda (ISOOCTANE) = 236nm( epsilon 27200) [[Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115|{{RelationTable/GetFirstAuthor|Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115}}]] | ||
|NMR Spectra=METHYL ESTER ; ^1 H-NMR(90MHz) : delta 6.56(dd, J=11, 14.5Hz, 1H, 13-CH), 6.02(dd, J=11,11Hz, 1H, 12-CH), 5.7(dd, J=6.8, 14.5Hz, 1H,14-CH), 5.51(dd, J=6,11Hz, 1H, 11-CH), 4.16(dd, J=6.8, 6.8Hz, 1H, 15-CH), 3.69(s, 3H, OCH_3 ), 2.98(2H, 10-CH), 2.84(2H, 7-CH), 2.35(2H, 2-CH), 2.08(2H, 4-CH), 1.47(2H, 16-CH), 2.3-0.9(11H) [[Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115|{{RelationTable/GetFirstAuthor|Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115}}]] | |||
|Source= | |||
|Chemical Synthesis=[[Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115|{{RelationTable/GetFirstAuthor|Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115}}]] {{Image200|LBF20407HO07FT0001.gif}} | |||
|Metabolism=When arachidonic acid is oxygenated by 15-lipoxygenase, 15(S)-hydroperoxy-5,8,11,13-(Z,Z,Z,E)-eicosatetraenoic acid is produced [[Reference:Bryant_RW:Bailey_JM:Schewe_T:Rapoport_SM:,J. Biol. Chem.,1982,257,6050|{{RelationTable/GetFirstAuthor|Reference:Bryant_RW:Bailey_JM:Schewe_T:Rapoport_SM:,J. Biol. Chem.,1982,257,6050}}]]. The latter compound is reduced to a corresponding 15(S)-hydroxy acid with whole cells or crude enzyme preparations. | |||
|Symbol=15S-HETE | |||
|Biological Activity=In connection with biological activities of 15-hydroxyeicosatetraenoic acid, there are reports for pain response of skin, mucus secretion in airway, histamine hypersensitivity, prolactin secretion, and regulation of protein phosporylation and cell signalling [[Reference:Kuhn_H:Thiele_BJ:,J. Lipid Mediat. Cell Signal.,1995,12,157|{{RelationTable/GetFirstAuthor|Reference:Kuhn_H:Thiele_BJ:,J. Lipid Mediat. Cell Signal.,1995,12,157}}]]. | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 16:35, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR6103 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20407HO07 |
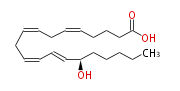
| 15S-Hydroxy- (5Z,8Z,11Z,13E) -eicosatetraenoic acid | |
|---|---|
| |
| Structural Information | |
| 15S-Hydroxy- (cis-5,cis-8,cis-11,trans-13) -eicosatetraenoic acid | |
| |
| 15S-HETE | |
| Formula | C20H32O3 |
| Exact Mass | 320.23514489 |
| Average Mass | 320.46628 |
| SMILES | C(CC[C@@H](C=CC=CCC=CCC=CCCCC(O)=O)O)CC |
| Physicochemical Information | |
| DIETHYL ETHER Baldwin_JE et al. | |
|
Baldwin_JE et al. | |
| When arachidonic acid is oxygenated by 15-lipoxygenase, 15(S)-hydroperoxy-5,8,11,13-(Z,Z,Z,E)-eicosatetraenoic acid is produced Bryant_RW et al.. The latter compound is reduced to a corresponding 15(S)-hydroxy acid with whole cells or crude enzyme preparations. | |
| In connection with biological activities of 15-hydroxyeicosatetraenoic acid, there are reports for pain response of skin, mucus secretion in airway, histamine hypersensitivity, prolactin secretion, and regulation of protein phosporylation and cell signalling Kuhn_H et al.. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER TMS ETHER ; m/e 406(M+), 391, 335, 316, 305, 225, 173 Baldwin_JE et al. |
| UV Spectra | METHYL ESTER ; λ (ISOOCTANE) = 236nm( ε 27200) Baldwin_JE et al. |
| IR Spectra | |
| NMR Spectra | METHYL ESTER ; 1H-NMR(90MHz) : δ 6.56(dd, J=11, 14.5Hz, 1H, 13-CH), 6.02(dd, J=11,11Hz, 1H, 12-CH), 5.7(dd, J=6.8, 14.5Hz, 1H,14-CH), 5.51(dd, J=6,11Hz, 1H, 11-CH), 4.16(dd, J=6.8, 6.8Hz, 1H, 15-CH), 3.69(s, 3H, OCH3), 2.98(2H, 10-CH), 2.84(2H, 7-CH), 2.35(2H, 2-CH), 2.08(2H, 4-CH), 1.47(2H, 16-CH), 2.3-0.9(11H) Baldwin_JE et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|