LBGPEccp:R:R:YS2ANe005: Difference between revisions
m (LBGPEccp:R:R:YS2ANe005:01 moved to LBGPEccp:R:R:YS2ANe005) |
m (LBGPEccp:R:R:YS2ANe005:01 moved to LBGPEccp:R:R:YS2ANe005) |
||
| Line 4: | Line 4: | ||
|LipidBank=PGP2410 | |LipidBank=PGP2410 | ||
|SysName=L-a-phosphatidylethanolamine | |SysName=L-a-phosphatidylethanolamine | ||
|Common Name= | |Common Name=phosphatidylethanolamine cephalin ethanolaminephosphoglyceride (3-sn-phosphatidyl) ethanolamine L-a-phosphatidylethanolamine | ||
|Melting Point=196°C | |Melting Point=196°C 2455; dimyristoyl-PE, 175-177°C 2456/2457; dipalmitoyl-PE, 172-175°C 2456/2457 210-211°C 2469 dipalmitoyl-DL-, 211°C 2469; distearoyl-PE, 172-173.5°C 2456/2457; dioleoyl-PE, 195-200°C 2469; dielaidoyl-PE, 193°C 2469; 1-palmitoyl-2-oleoyl-PE, 194-196°C 2471 | ||
|Reflactive=dimyristoyl-PE , [ | |Reflactive=dimyristoyl-PE , [FONT FACE=Symbola/FONT]subD/subsup26/sup+6.7° in CHClSUBFONT SIZE=-13/FONT/SUB 2456/2457; dipalmitoyl-PE, [FONT FACE=Symbola/FONT]subD/subsup26/sup+6.4° in CHClSUBFONT SIZE=-13/FONT/SUB 2456/2457, [FONT FACE=Symbola/FONT]subD/subsup25/sup+6.2° in CHClSUBFONT SIZE=-13/FONT/SUB-MeOH (2:1,v/v) 2469; distearoyl-PE, [FONT FACE=Symbola/FONT]subD/subsup24/sup+6.0° in CHClSUBFONT SIZE=-13/FONT/SUB-glacial acetic acid (9:1,v/v) 2456/2457; dioleoyl-PE, [FONT FACE=Symbola/FONT]subD/subsup22/sup+6.0° in CHClSUBFONT SIZE=-13/FONT/SUB 2469; dielaidoyl-PE, [FONT FACE=Symbola/FONT]subD/subsup22/sup+6.1° in CHClSUBFONT SIZE=-13/FONT/SUB 2469; 1-palmitoyl-2-oleoyl-PE, [FONT FACE=Symbola/FONT]subD/subsup22/sup+6.35° in CHClSUBFONT SIZE=-13/FONT/SUB 2469 | ||
|Solubility=Insoluble in water. Natural PE is soluble in MeOH, EtOH, | |Solubility=Insoluble in water. Natural PE is soluble in MeOH, EtOH, CHClSUBFONT SIZE=-13/FONT/SUB, benzene, ether, and petroleum ether, and insoluble in acetone. At 20°C, synthetic PEs are insoluble (=1mg/100 ml of dry solvent) in acetone, ether, petroleum ether, and ethyl acetate; moderately soluble (20-100 mg/100 ml of dry solvent) in EtOH, pyridine, benzene, and CClSUBFONT SIZE=-14/FONT/SUB; readily soluble ( 1g/100 ml of solvent) in CHClSUBFONT SIZE=-13/FONT/SUB 2455/2456/2457. | ||
|Mass Spectra=Negative ion mass spectrum during the elution of 1-stearol-2-arachidonyl-PE in a sample of rat liver PE. A Fisons VG Platform II single quadrupole mass spectrometer, fitted with an electrospray ion source operated at atmospheric pressure, was used for the identification of components eluting from the column. Nitrogen was used as nebulizer gas and as curtain gas. The capillary voltage was set to 3.0 kV and the cone voltage to 100 V {{Image200|LBGPEccp:R:R: | |Mass Spectra=Negative ion mass spectrum during the elution of 1-stearol-2-arachidonyl-PE in a sample of rat liver PE. A Fisons VG Platform II single quadrupole mass spectrometer, fitted with an electrospray ion source operated at atmospheric pressure, was used for the identification of components eluting from the column. Nitrogen was used as nebulizer gas and as curtain gas. The capillary voltage was set to 3.0 kV and the cone voltage to 100 V {{Image200|LBGPEccp:R:R:YS2ANe005SP0004.gif}} 2462., | ||
|UV Spectra=Maximum absorbance at 205 nm | |UV Spectra=Maximum absorbance at 205 nm 2458. | ||
|IR Spectra=Fourier transform IR spectra for dimyristoyl-PE in the dry state (anhydrous), either in the pure state (A, C and E) or in the presence of 20 mol% of | |IR Spectra=Fourier transform IR spectra for dimyristoyl-PE in the dry state (anhydrous), either in the pure state (A, C and E) or in the presence of 20 mol% of FONT FACE=Symbola/FONT-tocopherol (B, D and F). Ester C=O stretching vibration mode region,1800-1700 cmSUPFONT SIZE=-1-/FONT/SUPSUPFONT SIZE=-11/FONT/SUP; CHSUBFONT SIZE=-12/FONT/SUB deformation, scissoring band region, 1500-1400 cmSUPFONT SIZE=-1-/FONT/SUPSUPFONT SIZE=-11/FONT/SUP; headgroup POSUBFONT SIZE=-12/FONT/SUBSUPFONT SIZE=-1-/FONT/SUP phosphate band region, 1350-1150 cmSUPFONT SIZE=-1-/FONT/SUPSUPFONT SIZE=-11/FONT/SUP {{Image200|LBGPEccp:R:R:YS2ANe005SP0001.gif}} 2459. | ||
|NMR Spectra= | |NMR Spectra=SUPFONT SIZE=-11/FONT/SUPH-NMR spectrum of PE. Varian VXR500 spectrometer operating at 500 MHz for protons, using CHClSUBFONT SIZE=-13/FONT/SUB as the lock signal {{Image200|LBGPEccp:R:R:YS2ANe005SP0002.gif}} 2460./BRSUPFONT SIZE=-13/FONT/SUPSUPFONT SIZE=-11/FONT/SUPP NMR spectrum of a PE (left) and a PC (right) fraction from bovine brain. Varian Unity 300 spectrometer operating at121.42 MHz using 1% HSUBFONT SIZE=-13/FONT/SUBPOSUBFONT SIZE=-14/FONT/SUB as external standard {{Image200|LBGPEccp:R:R:YS2ANe005SP0003.gif}} 2461., | ||
|Chromatograms=2 dimensional TLC {{Image200|LBGPEccp:R:R: | |Chromatograms=2 dimensional TLC {{Image200|LBGPEccp:R:R:YS2ANe005CH0001.gif}} 2463/BRHPLC, Hewlett-Packard Model 1050 HPLC system, UV absorbance 205nm, Hewlett-Packard Model 3396 series II integrator, HP Hypersil C18 (200 x 2.1x 5 FONT FACE=Symbolm/FONTm) column {{Image200|LBGPEccp:R:R:YS2ANe005CH0002.gif}} 2464/BR HPLC for intact PE molecular species. Molecular species of approxymately 50 nmol of PE were separated on two 250 x 4 mm Lichrospher RP-18 endcapped columns in series (Merck, Darmstadt, Germany). Isocratic elution was performed with a solvent consisting of acetonitrile-methanol 3:7 (v/v) containing 5 FONT FACE=Symbolm/FONTM ethanolamine, or a solvent consisiting of acetonitrile-methanol 2:3 (v/v) containing 2 FONT FACE=Symbolm/FONTM ethanolamine at a flow rate of 1.25 ml/min. The column effuluent was monitored at 206 nm using LKB 2251 Uvicord (Pharmacia, Upsala, Sweden) and subsequent ELSD was performed using a Varex MKIII obtained from Alltech, (Deerfield, IL) operating at a gas flow rate of 1.9 l/ml and a drift tube temperature of 100°C {{Image200|LBGPEccp:R:R:YS2ANe005CH0003.gif}} {{Image200|LBGPEccp:R:R:YS2ANe005FT0001.gif}} 2462., | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 20:00, 27 January 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | PGP2410 |
| LipidMaps | [1] |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBGPEccp:R:R:YS2ANe005 |
| GlcNAca/b1-3Xyla-4Galb1-3GalNAca1-4(NeuAc?1-2NeuGc4Mea1-3)GalNAcb1-4(EtnP-6)GlcNAcb1-3Manb1-4Glcb1-1Cer | |
|---|---|
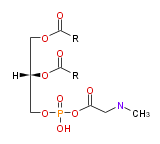
| |
| Structural Information | |
| L-a-phosphatidylethanolamine | |
| |
| Formula | |
| Exact Mass | |
| Average Mass | |
| SMILES | |
| Physicochemical Information | |
| 196°C 2455; dimyristoyl-PE, 175-177°C 2456/2457; dipalmitoyl-PE, 172-175°C 2456/2457 210-211°C 2469 dipalmitoyl-DL-, 211°C 2469; distearoyl-PE, 172-173.5°C 2456/2457; dioleoyl-PE, 195-200°C 2469; dielaidoyl-PE, 193°C 2469; 1-palmitoyl-2-oleoyl-PE, 194-196°C 2471 | |
| Insoluble in water. Natural PE is soluble in MeOH, EtOH, CHClSUBFONT SIZE=-13/FONT/SUB, benzene, ether, and petroleum ether, and insoluble in acetone. At 20°C, synthetic PEs are insoluble (=1mg/100 ml of dry solvent) in acetone, ether, petroleum ether, and ethyl acetate; moderately soluble (20-100 mg/100 ml of dry solvent) in EtOH, pyridine, benzene, and CClSUBFONT SIZE=-14/FONT/SUB; readily soluble ( 1g/100 ml of solvent) in CHClSUBFONT SIZE=-13/FONT/SUB 2455/2456/2457. | |
| Spectral Information | |
| Mass Spectra | Negative ion mass spectrum during the elution of 1-stearol-2-arachidonyl-PE in a sample of rat liver PE. A Fisons VG Platform II single quadrupole mass spectrometer, fitted with an electrospray ion source operated at atmospheric pressure, was used for the identification of components eluting from the column. Nitrogen was used as nebulizer gas and as curtain gas. The capillary voltage was set to 3.0 kV and the cone voltage to 100 V 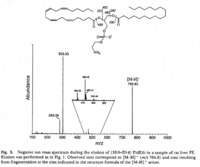 2462., |
| UV Spectra | Maximum absorbance at 205 nm 2458. |
| IR Spectra | Fourier transform IR spectra for dimyristoyl-PE in the dry state (anhydrous), either in the pure state (A, C and E) or in the presence of 20 mol% of FONT FACE=Symbola/FONT-tocopherol (B, D and F). Ester C=O stretching vibration mode region,1800-1700 cmSUPFONT SIZE=-1-/FONT/SUPSUPFONT SIZE=-11/FONT/SUP; CHSUBFONT SIZE=-12/FONT/SUB deformation, scissoring band region, 1500-1400 cmSUPFONT SIZE=-1-/FONT/SUPSUPFONT SIZE=-11/FONT/SUP; headgroup POSUBFONT SIZE=-12/FONT/SUBSUPFONT SIZE=-1-/FONT/SUP phosphate band region, 1350-1150 cmSUPFONT SIZE=-1-/FONT/SUPSUPFONT SIZE=-11/FONT/SUP 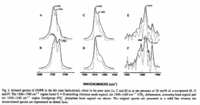 2459. |
| NMR Spectra | SUPFONT SIZE=-11/FONT/SUPH-NMR spectrum of PE. Varian VXR500 spectrometer operating at 500 MHz for protons, using CHClSUBFONT SIZE=-13/FONT/SUB as the lock signal 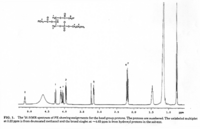 2460./BRSUPFONT SIZE=-13/FONT/SUPSUPFONT SIZE=-11/FONT/SUPP NMR spectrum of a PE (left) and a PC (right) fraction from bovine brain. Varian Unity 300 spectrometer operating at121.42 MHz using 1% HSUBFONT SIZE=-13/FONT/SUBPOSUBFONT SIZE=-14/FONT/SUB as external standard 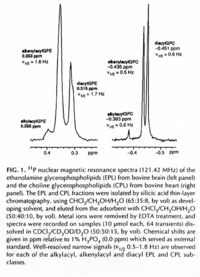 2461., |
| Other Spectra | |
| Chromatograms | 2 dimensional TLC 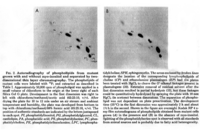 2463/BRHPLC, Hewlett-Packard Model 1050 HPLC system, UV absorbance 205nm, Hewlett-Packard Model 3396 series II integrator, HP Hypersil C18 (200 x 2.1x 5 FONT FACE=Symbolm/FONTm) column 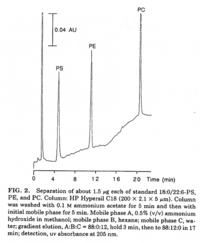 2464/BR HPLC for intact PE molecular species. Molecular species of approxymately 50 nmol of PE were separated on two 250 x 4 mm Lichrospher RP-18 endcapped columns in series (Merck, Darmstadt, Germany). Isocratic elution was performed with a solvent consisting of acetonitrile-methanol 3:7 (v/v) containing 5 FONT FACE=Symbolm/FONTM ethanolamine, or a solvent consisiting of acetonitrile-methanol 2:3 (v/v) containing 2 FONT FACE=Symbolm/FONTM ethanolamine at a flow rate of 1.25 ml/min. The column effuluent was monitored at 206 nm using LKB 2251 Uvicord (Pharmacia, Upsala, Sweden) and subsequent ELSD was performed using a Varex MKIII obtained from Alltech, (Deerfield, IL) operating at a gas flow rate of 1.9 l/ml and a drift tube temperature of 100°C 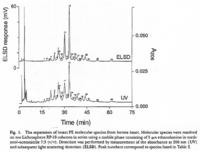 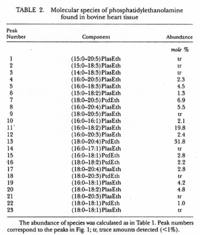 2462., |