LBF18206HP01: Difference between revisions
No edit summary |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 7: | Line 7: | ||
|LipidMaps=LMFA01040004 | |LipidMaps=LMFA01040004 | ||
|SysName=9-Hydroperoxy-10,12-octadecadienoic acid | |SysName=9-Hydroperoxy-10,12-octadecadienoic acid | ||
|Common Name=&&9- | |Common Name=&&9-HPODE&& | ||
|Mass Spectra=GC/EI-MS(after methanolysis, reduction and trimethylsilylation) [[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908}}]][[Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31|{{RelationTable/GetFirstAuthor|Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31}}]][[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]][[Reference:Frankel_EN:Neff_WE:Bessler_TR:,Lipids,1979,14,961|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Bessler_TR:,Lipids,1979,14,961}}]][[Reference:Hamberg_M:,Lipids,1975,10,87|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Lipids,1975,10,87}}]]: m/e= 382[M], 292[M-HOTMS], 311[M-(CH2)4CH3], 225[M-(CH2)7COOCH3] standard peak/ GC-EI-MS(after methylation, reduction and hydrogenation) [[Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100}}]][[Reference:Dolev_A:Rohwedder_WK:Dutton_HJ:,Lipids,1967,2,28|{{RelationTable/GetFirstAuthor|Reference:Dolev_A:Rohwedder_WK:Dutton_HJ:,Lipids,1967,2,28}}]][[Reference:Zimmerman_DC:Vick_BA:,Lipids,1970,5,392|{{RelationTable/GetFirstAuthor|Reference:Zimmerman_DC:Vick_BA:,Lipids,1970,5,392}}]]: m/e= 187[CH(OH)(CH2)7COOCH3], 158[(CH2)7COOCH3+H], 155[C(OH)-(CH)7CO] | |Mass Spectra=GC/EI-MS(after methanolysis, reduction and trimethylsilylation) [[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908}}]][[Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31|{{RelationTable/GetFirstAuthor|Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31}}]][[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]][[Reference:Frankel_EN:Neff_WE:Bessler_TR:,Lipids,1979,14,961|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Bessler_TR:,Lipids,1979,14,961}}]][[Reference:Hamberg_M:,Lipids,1975,10,87|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Lipids,1975,10,87}}]]: m/e= 382[M], 292[M-HOTMS], 311[M-(CH2)4CH3], 225[M-(CH2)7COOCH3] standard peak/ GC-EI-MS(after methylation, reduction and hydrogenation) [[Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100}}]][[Reference:Dolev_A:Rohwedder_WK:Dutton_HJ:,Lipids,1967,2,28|{{RelationTable/GetFirstAuthor|Reference:Dolev_A:Rohwedder_WK:Dutton_HJ:,Lipids,1967,2,28}}]][[Reference:Zimmerman_DC:Vick_BA:,Lipids,1970,5,392|{{RelationTable/GetFirstAuthor|Reference:Zimmerman_DC:Vick_BA:,Lipids,1970,5,392}}]]: m/e= 187[CH(OH)(CH2)7COOCH3], 158[(CH2)7COOCH3+H], 155[C(OH)-(CH)7CO] | ||
|UV Spectra=Trans, cis isomer: lambda max=236nm, epsilon =25900, trans, trans isomer: lambda max=233nm, epsilon =28600 [[Reference:Chan_HW:Levett_G:,Lipids,1977,12,99|{{RelationTable/GetFirstAuthor|Reference:Chan_HW:Levett_G:,Lipids,1977,12,99}}]][[Reference:Bolland_JL:Koch_HP:,J. Chem. Soc.,1945,,445|{{RelationTable/GetFirstAuthor|Reference:Bolland_JL:Koch_HP:,J. Chem. Soc.,1945,,445}}]][[Reference:Lundberg_WO:Chipault_JR:,J. Am. Chem. Soc.,1947,69,833|{{RelationTable/GetFirstAuthor|Reference:Lundberg_WO:Chipault_JR:,J. Am. Chem. Soc.,1947,69,833}}]][[Reference:Lundberg_WO:Chipault_JR:Hendrickson_NJ:,J. Am. Oil Chem. Soc.,1949,26,109|{{RelationTable/GetFirstAuthor|Reference:Lundberg_WO:Chipault_JR:Hendrickson_NJ:,J. Am. Oil Chem. Soc.,1949,26,109}}]][[Reference:Gardner_HW:Weisleder_D:,Lipids,1970,5,678|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1970,5,678}}]][[Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191}}]] | |UV Spectra=Trans, cis isomer: lambda max=236nm, epsilon =25900, trans, trans isomer: lambda max=233nm, epsilon =28600 [[Reference:Chan_HW:Levett_G:,Lipids,1977,12,99|{{RelationTable/GetFirstAuthor|Reference:Chan_HW:Levett_G:,Lipids,1977,12,99}}]][[Reference:Bolland_JL:Koch_HP:,J. Chem. Soc.,1945,,445|{{RelationTable/GetFirstAuthor|Reference:Bolland_JL:Koch_HP:,J. Chem. Soc.,1945,,445}}]][[Reference:Lundberg_WO:Chipault_JR:,J. Am. Chem. Soc.,1947,69,833|{{RelationTable/GetFirstAuthor|Reference:Lundberg_WO:Chipault_JR:,J. Am. Chem. Soc.,1947,69,833}}]][[Reference:Lundberg_WO:Chipault_JR:Hendrickson_NJ:,J. Am. Oil Chem. Soc.,1949,26,109|{{RelationTable/GetFirstAuthor|Reference:Lundberg_WO:Chipault_JR:Hendrickson_NJ:,J. Am. Oil Chem. Soc.,1949,26,109}}]][[Reference:Gardner_HW:Weisleder_D:,Lipids,1970,5,678|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1970,5,678}}]][[Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191}}]] | ||
| Line 16: | Line 16: | ||
|Metabolism= | |Metabolism= | ||
|Biological Activity=Pysiological damages are induced by these hydroperoxides which are incorporated into bodies or synthesized endogenously.[[Reference:Logani_MK:Davies_RE:,Lipids,1980,15,485|{{RelationTable/GetFirstAuthor|Reference:Logani_MK:Davies_RE:,Lipids,1980,15,485}}]]<!--8044--><!--8045-->[[Reference:Sevanian_A:Hochstein_P:,Annu. Rev. Nutr.,1985,5,365|{{RelationTable/GetFirstAuthor|Reference:Sevanian_A:Hochstein_P:,Annu. Rev. Nutr.,1985,5,365}}]][[Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21|{{RelationTable/GetFirstAuthor|Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21}}]]<!--8048--><!--8049--> | |Biological Activity=Pysiological damages are induced by these hydroperoxides which are incorporated into bodies or synthesized endogenously.[[Reference:Logani_MK:Davies_RE:,Lipids,1980,15,485|{{RelationTable/GetFirstAuthor|Reference:Logani_MK:Davies_RE:,Lipids,1980,15,485}}]]<!--8044--><!--8045-->[[Reference:Sevanian_A:Hochstein_P:,Annu. Rev. Nutr.,1985,5,365|{{RelationTable/GetFirstAuthor|Reference:Sevanian_A:Hochstein_P:,Annu. Rev. Nutr.,1985,5,365}}]][[Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21|{{RelationTable/GetFirstAuthor|Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21}}]]<!--8048--><!--8049--> | ||
}} | |||
{{MassbankSpectra| | |||
UT000253 | |||
UT000254 | |||
UT000255 | |||
UT000256 | |||
UT000257 | |||
UT000258 | |||
UT000259 | |||
UT000260 | |||
UT000261 | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 09:00, 17 January 2014
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8001 |
| LipidMaps | LMFA01040004 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18206HP01 |
| 9-HPODE | |
|---|---|
| |
| Structural Information | |
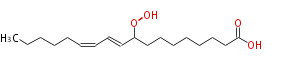
| 9-Hydroperoxy-10,12-octadecadienoic acid | |
| |
| Formula | C18H32O4 |
| Exact Mass | 312.23005951199997 |
| Average Mass | 312.44428 |
| SMILES | CCCCCC=CC=CC(OO)CCCCCCCC(O)=O |
| Physicochemical Information | |
| Auto oxidation of methyllinoleate Frankel_EN Frankel_EN Frankel_EN Frankel_EN Chan_HWS et al.. Oxidation of methyl linoleate by singlet oxygen Frankel_EN Frankel_EN Frankel_EN Frankel_EN . Oxidation of linoleic acid by lipoxygenase Mathuo_M Wakabayashi_T . Production mechanism (auto oxidation): bis-allylic hydrogen at C11. | |
| Pysiological damages are induced by these hydroperoxides which are incorporated into bodies or synthesized endogenously. Logani_MK et al. Sevanian_A et al. Fujimoto_K | |
| Spectral Information | |
| Mass Spectra | GC/EI-MS(after methanolysis, reduction and trimethylsilylation) Frankel_EN et al. KleimanRet al. Gardner_HW et al. Frankel_EN et al. HambergM: m/e= 382[M], 292[M-HOTMS], 311[M-(CH2)4CH3], 225[M-(CH2)7COOCH3] standard peak/ GC-EI-MS(after methylation, reduction and hydrogenation) Chan_HWS DolevAet al. Zimmerman_DC et al.: m/e= 187[CH(OH)(CH2)7COOCH3], 158[(CH2)7COOCH3+H], 155[C(OH)-(CH)7CO] |
| UV Spectra | Trans, cis isomer: λ max=236nm, ε =25900, trans, trans isomer: λ max=233nm, ε =28600 Chan_HW et al. Bolland_JL et al. Lundberg_WO et al. Lundberg_WO et al. Gardner_HW et al. Gardner_HW et al. |
| IR Spectra | Methyl ester: Chan_HW et al. Gardner_HW et al. Cannon_JA et al. Privett_OS et al. Sephton_HH et al. Graveland_A_ Privett_OS et al. Gardner_HW et al.: trans, cis isomer: 986 and 949cm-1, trans, trans isomer: 989cm-1, OOH group: 3550cm-1 |
| NMR Spectra | 1H-NMR Chan_HW et al. Frankel_EN et al., 1H-NMR( after methanolyzation and reduction) Gardner_HW et al. Neff_WE et al.: trans,cis isomer: C10-13 (5.42-6.48ppm), C14 (2.10-2.18ppm), C9(4.15ppm), J10-11= 15.4Hz(trans), J12-13= 10.8Hz (cis), trans, trans isomer: olefinic protons (5.41ppm), C14 (2.07ppm), C9 (4.20ppm) |
| Other Spectra | |
| Chromatograms | |








