LBF18303HP01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 15: | Line 15: | ||
|Metabolism= | |Metabolism= | ||
|Biological Activity=Pysiological damages are induced by these hydroperoxides which are incorporated into bodies or synthesized endogenously.[[Reference:Logani_MK:Davies_RE:,Lipids,1980,15,485|{{RelationTable/GetFirstAuthor|Reference:Logani_MK:Davies_RE:,Lipids,1980,15,485}}]]<!--8044--><!--8045-->[[Reference:Sevanian_A:Hochstein_P:,Annu. Rev. Nutr.,1985,5,365|{{RelationTable/GetFirstAuthor|Reference:Sevanian_A:Hochstein_P:,Annu. Rev. Nutr.,1985,5,365}}]][[Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21|{{RelationTable/GetFirstAuthor|Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21}}]]<!--8048--><!--8049-->. It reacts with DNA in the presence of Fe ions and ascorbic acid[[Reference:Fujimoto_K:Neff_WE:Frankel_EN:,Biochim. Biophys. Acta,1984,795,100|{{RelationTable/GetFirstAuthor|Reference:Fujimoto_K:Neff_WE:Frankel_EN:,Biochim. Biophys. Acta,1984,795,100}}]]. | |Biological Activity=Pysiological damages are induced by these hydroperoxides which are incorporated into bodies or synthesized endogenously.[[Reference:Logani_MK:Davies_RE:,Lipids,1980,15,485|{{RelationTable/GetFirstAuthor|Reference:Logani_MK:Davies_RE:,Lipids,1980,15,485}}]]<!--8044--><!--8045-->[[Reference:Sevanian_A:Hochstein_P:,Annu. Rev. Nutr.,1985,5,365|{{RelationTable/GetFirstAuthor|Reference:Sevanian_A:Hochstein_P:,Annu. Rev. Nutr.,1985,5,365}}]][[Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21|{{RelationTable/GetFirstAuthor|Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21}}]]<!--8048--><!--8049-->. It reacts with DNA in the presence of Fe ions and ascorbic acid[[Reference:Fujimoto_K:Neff_WE:Frankel_EN:,Biochim. Biophys. Acta,1984,795,100|{{RelationTable/GetFirstAuthor|Reference:Fujimoto_K:Neff_WE:Frankel_EN:,Biochim. Biophys. Acta,1984,795,100}}]]. | ||
}} | |||
{{MassbankSpectra| | |||
UT000262 | |||
UT000263 | |||
UT000264 | |||
UT000265 | |||
UT000266 | |||
UT000267 | |||
UT000268 | |||
UT000269 | |||
UT000270 | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 09:00, 17 January 2014
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8050 |
| LipidMaps | LMFA01040013 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18303HP01 |
| 9-HpOTrE | |
|---|---|
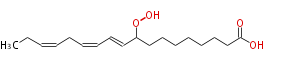
| |
| Structural Information | |
| 9-Hydroperoxy-10,12,15-octadecatrienoic acid | |
| |
| Formula | C18H30O4 |
| Exact Mass | 310.21440944799997 |
| Average Mass | 310.4284 |
| SMILES | CCC=CCC=CC=CC(OO)CCCCCCCC(O)=O |
| Physicochemical Information | |
| Auto oxidation of linoleate Terao_J et al. Frankel_EN Frankel_EN Frankel_EN Chan_HWS et al.. Oxidation of linoleate by singlet oxygen Frankel_EN Frankel_EN Frankel_EN . Reaction products between linoleate and linseed lipoxygenase(pH6.5, 24°C) Zimmerman_DC et al.. | |
| Pysiological damages are induced by these hydroperoxides which are incorporated into bodies or synthesized endogenously. Logani_MK et al. Sevanian_A et al. Fujimoto_K . It reacts with DNA in the presence of Fe ions and ascorbic acid Fujimoto_K et al.. | |
| Spectral Information | |
| Mass Spectra | EI-MS(Me-ester; after reduction and hydrogenation) Chan_HWS Chan_HWS et al.: m/e=187[O=CH(CH2)7C(=OH)OCH3]; 158[CH2(CH2)6C(=OH)OCH3]; 155[O=CH(CH2)7C=O], GC-EI-MS(Me-ester; after reduction and TMS) TeraoJet al. Frankel_EN et al.: m/e=380[M]; 365[M-CH3]; 223[SMTO=CH-CH=CH-CH=CH-CH2-CH=CH-CH2CH3] |
| UV Spectra | (Me-ester; after reduction; in ethanol) Chan_HWS et al., trans, cis, cis isomer: λ max=236nm trans, trans, cis isomer: λ max=232nm |
| IR Spectra | (Me-ester; after reduction) Chan_HWS et al. Frankel_EN et al., trans, cis, cis isomer:9 88-983 AND 951-945cm-1; trans, trans, cis isomer: 992-983cm-1 (Me-ester) ToyodaIet al., OOH group: 3400cm-1 |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|








