LBF20207PG22: Difference between revisions
No edit summary |
No edit summary |
||
| (17 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=XPR1301 | |LipidBank=XPR1301 | ||
|LipidMaps=LMFA03010004 | |LipidMaps=LMFA03010004 | ||
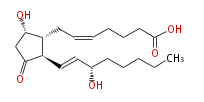
|SysName=7- [ | |SysName=7- [5S -Hydroxy-2R - (3S-hydroxy-trans-1-octenyl) -3-oxocyclopentan-1R -yl] -cis-5-heptenoic acid | ||
|Common Name=&& | |Common Name=&&Prostaglandin D_2&&7- [ 5 (S) -Hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) -3-oxocyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid&& | ||
|Melting Point=68°C [[Reference:Hayashi_M:Tanouchi_T:,J. Org. Chem.,1973,38,2115|{{RelationTable/GetFirstAuthor|Reference:Hayashi_M:Tanouchi_T:,J. Org. Chem.,1973,38,2115}}]] | |Melting Point=68°C [[Reference:Hayashi_M:Tanouchi_T:,J. Org. Chem.,1973,38,2115|{{RelationTable/GetFirstAuthor|Reference:Hayashi_M:Tanouchi_T:,J. Org. Chem.,1973,38,2115}}]] | ||
| | |Optical=[ alpha ]^{20}_D =9° (C=2.11,THF) [[Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625|{{RelationTable/GetFirstAuthor|Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625}}]] | ||
|Solubility=ETHYL ACETATE,THF,CHLOROFORM [[Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625|{{RelationTable/GetFirstAuthor|Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625}}]] | |Solubility=ETHYL ACETATE,THF,CHLOROFORM [[Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625|{{RelationTable/GetFirstAuthor|Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625}}]] | ||
|Mass Spectra=m/e 334, 316, 246, 245, 191, 190, 161, 55 [[Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625|{{RelationTable/GetFirstAuthor|Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625}}]] | |Mass Spectra=m/e 334, 316, 246, 245, 191, 190, 161, 55 [[Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625|{{RelationTable/GetFirstAuthor|Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625}}]] | ||
|IR Spectra=KBr : 3400-2500, 1740, 1700, 975cm | |IR Spectra=KBr : 3400-2500, 1740, 1700, 975cm^{-1} [[Reference:Hayashi_M:Tanouchi_T:,J. Org. Chem.,1973,38,2115|{{RelationTable/GetFirstAuthor|Reference:Hayashi_M:Tanouchi_T:,J. Org. Chem.,1973,38,2115}}]] | ||
|NMR Spectra= | |NMR Spectra=^1 H-NMR(270MHz, CDCl_3 ) : delta 5.63(dd,J=7 & 16Hz,1H,14-CH), 5.43(dd,J=16 & 8.5Hz,1H,13-CH), 4.47(m, 1H, 9-CH), 4.09(bq, J=7Hz, 1H, 15-CH), 2.87(dd, J=12 & 8.5Hz, 1H, 12-CH), 2.45(m, 2H, 10-CH2), 1.95(bm, 1H, 8-CH) [[Reference:Jenny_EF:Schaeublin_P:,Tetrah. Lett.,1974,,2235|{{RelationTable/GetFirstAuthor|Reference:Jenny_EF:Schaeublin_P:,Tetrah. Lett.,1974,,2235}}]] | ||
|Source=In most animal tissues prostanoids are synthesized enzymatically de novo upon physiological and pathological stimulations, and this is also the case of prostaglandin D2. Prostaglandin D2 is produced in barin, lung, spleen, mast cells and other tissues [[Reference:Urade_Y:Watanabe_K:Hayaishi_O:,J. Lipid Mediat. Cell Signal.,1995,12,257|{{RelationTable/GetFirstAuthor|Reference:Urade_Y:Watanabe_K:Hayaishi_O:,J. Lipid Mediat. Cell Signal.,1995,12,257}}]]. | |||
|Chemical Synthesis=[[Reference:Hayashi_M:Tanouchi_T:,J. Org. Chem.,1973,38,2115|{{RelationTable/GetFirstAuthor|Reference:Hayashi_M:Tanouchi_T:,J. Org. Chem.,1973,38,2115}}]] {{Image200|LBF20207PG22FT0001.gif}} | |||
|Metabolism=Prostaglandin D2 is produced by isomerization of 9,11-endoperoxide of prostaglandin H2. There are two isozymes of prostaglandin D synthase (hematopoietic type and lipocalin type) distinguished by glutathione reequirement and effect of inhibitor [[Reference:Urade_Y:Watanabe_K:Hayaishi_O:,J. Lipid Mediat. Cell Signal.,1995,12,257|{{RelationTable/GetFirstAuthor|Reference:Urade_Y:Watanabe_K:Hayaishi_O:,J. Lipid Mediat. Cell Signal.,1995,12,257}}]]. Prostaglandin D2 is either reduced to 9 alpha ,11 beta -prostaglandin F or oxidized to 15-keto-prostaglandin D2 by a specific dehydrogenase [[Reference:Giles_H:Leff_P:,Prostaglandins,1988,35,277|{{RelationTable/GetFirstAuthor|Reference:Giles_H:Leff_P:,Prostaglandins,1988,35,277}}]]. Non-enzymatic transformation of prostaglandin D2 produces anti-neoplastic Delta 12-prostaglandin J2 [[Reference:Negishi_M:Koizumi_T:Ichikawa_A:,J. Lipid Mediat. Cell Signal.,1995,12,443|{{RelationTable/GetFirstAuthor|Reference:Negishi_M:Koizumi_T:Ichikawa_A:,J. Lipid Mediat. Cell Signal.,1995,12,443}}]]. | |||
|Symbol=PGD2 | |||
|Biological Activity=Biological significance of prostaglandin D2 was unknown except anti-aggregatory and bronchoconstrictive activities [[Reference:Giles_H:Leff_P:,Prostaglandins,1988,35,277|{{RelationTable/GetFirstAuthor|Reference:Giles_H:Leff_P:,Prostaglandins,1988,35,277}}]]. Recently prostaglandin D2 has been noted as antineoplastic agent (actually attributed to Delta 12-prostaglandin J2) [[Reference:Fukushima_M:,Prostaglandins Leukot. Essent. Fatty Acids,1992,47,1|{{RelationTable/GetFirstAuthor|Reference:Fukushima_M:,Prostaglandins Leukot. Essent. Fatty Acids,1992,47,1}}]][[Reference:Negishi_M:Koizumi_T:Ichikawa_A:,J. Lipid Mediat. Cell Signal.,1995,12,443|{{RelationTable/GetFirstAuthor|Reference:Negishi_M:Koizumi_T:Ichikawa_A:,J. Lipid Mediat. Cell Signal.,1995,12,443}}]] and as a sleep-inducing compound [[Reference:Hayaishi_O:,J. Biol. Chem.,1988,263,14593|{{RelationTable/GetFirstAuthor|Reference:Hayaishi_O:,J. Biol. Chem.,1988,263,14593}}]]. Prostaglandin D2 binds to a receptor with 7 transmembrane domains (DP) coupled to a Gs protein [[Reference:Negishi_M:Sugimoto_Y:Ichikawa_A:,Biochim. Biophys. Acta,1995,1259,109|{{RelationTable/GetFirstAuthor|Reference:Negishi_M:Sugimoto_Y:Ichikawa_A:,Biochim. Biophys. Acta,1995,1259,109}}]]. | |||
|Genetic Information=cDNA and genomic DNA of prostaglandin D synthase were cloned [[Reference:Urade_Y:Watanabe_K:Hayaishi_O:,J. Lipid Mediat. Cell Signal.,1995,12,257|{{RelationTable/GetFirstAuthor|Reference:Urade_Y:Watanabe_K:Hayaishi_O:,J. Lipid Mediat. Cell Signal.,1995,12,257}}]]. cDNA for DP was isolated [[Reference:Negishi_M:Koizumi_T:Ichikawa_A:,J. Lipid Mediat. Cell Signal.,1995,12,443|{{RelationTable/GetFirstAuthor|Reference:Negishi_M:Koizumi_T:Ichikawa_A:,J. Lipid Mediat. Cell Signal.,1995,12,443}}]]. | |||
}} | |||
{{MassbankSpectra| | |||
UT000334 | |||
UT000335 | |||
UT000336 | |||
UT000337 | |||
UT000338 | |||
UT000339 | |||
UT000340 | |||
UT000341 | |||
UT000342 | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 09:00, 17 January 2014
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1301 |
| LipidMaps | LMFA03010004 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG22 |
| Prostaglandin D2 | |
|---|---|
| |
| Structural Information | |
| 7- [5S -Hydroxy-2R - (3S-hydroxy-trans-1-octenyl) -3-oxocyclopentan-1R -yl] -cis-5-heptenoic acid | |
| |
| PGD2 | |
| Formula | C20H32O5 |
| Exact Mass | 352.224974134 |
| Average Mass | 352.46508 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]1CC=CCCCC(O)=O)C(C[C@@H]1O)=O)CC |
| Physicochemical Information | |
| 68°C Hayashi_M et al. | |
| [ α ]20 D =9° (C=2.11,THF) OgawaYet al. | |
| ETHYL ACETATE,THF,CHLOROFORM OgawaYet al. | |
| In most animal tissues prostanoids are synthesized enzymatically de novo upon physiological and pathological stimulations, and this is also the case of prostaglandin D2. Prostaglandin D2 is produced in barin, lung, spleen, mast cells and other tissues Urade_Y et al.. | |
|
Hayashi_M et al. | |
| Prostaglandin D2 is produced by isomerization of 9,11-endoperoxide of prostaglandin H2. There are two isozymes of prostaglandin D synthase (hematopoietic type and lipocalin type) distinguished by glutathione reequirement and effect of inhibitor Urade_Y et al.. Prostaglandin D2 is either reduced to 9 alpha ,11 beta -prostaglandin F or oxidized to 15-keto-prostaglandin D2 by a specific dehydrogenase Giles_H et al.. Non-enzymatic transformation of prostaglandin D2 produces anti-neoplastic Delta 12-prostaglandin J2 Negishi_M et al.. | |
| Biological significance of prostaglandin D2 was unknown except anti-aggregatory and bronchoconstrictive activities Giles_H et al.. Recently prostaglandin D2 has been noted as antineoplastic agent (actually attributed to Delta 12-prostaglandin J2) Fukushima_M Negishi_M et al. and as a sleep-inducing compound Hayaishi_O . Prostaglandin D2 binds to a receptor with 7 transmembrane domains (DP) coupled to a Gs protein Negishi_M et al.. | |
| cDNA and genomic DNA of prostaglandin D synthase were cloned Urade_Y et al.. cDNA for DP was isolated Negishi_M et al.. | |
| Spectral Information | |
| Mass Spectra | m/e 334, 316, 246, 245, 191, 190, 161, 55 OgawaYet al. |
| UV Spectra | |
| IR Spectra | KBr : 3400-2500, 1740, 1700, 975cm-1 HayashiMet al. |
| NMR Spectra | 1H-NMR(270MHz, CDCl3) : δ 5.63(dd,J=7 & 16Hz,1H,14-CH), 5.43(dd,J=16 & 8.5Hz,1H,13-CH), 4.47(m, 1H, 9-CH), 4.09(bq, J=7Hz, 1H, 15-CH), 2.87(dd, J=12 & 8.5Hz, 1H, 12-CH), 2.45(m, 2H, 10-CH2), 1.95(bm, 1H, 8-CH) Jenny_EF et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|








