LBF20307PG38: Difference between revisions
No edit summary |
No edit summary |
||
| (24 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=XPR1901 | |LipidBank=XPR1901 | ||
|LipidMaps=LMFA03010019 | |LipidMaps=LMFA03010019 | ||
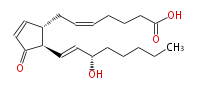
|SysName=7- [ | |SysName=7- [2R- (3S-hydroxy-trans-1-octenyl) -3-oxo-4-cyclopenten-1R-yl] -cis-5-heptenoic acid | ||
|Common Name=&&Prostaglandin J_2&&7- [2R- (3S-Hydroxy-1-(E)-octenyl) -3-oxo-4-cyclopenten-1R-yl] -5-(Z)-heptenoic acid&& | |||
|Solubility=ACETONITRILE< | |Solubility=ACETONITRILE<!--以下1048-->[[Reference:Mubarik-Ali_S:Chapeleo_CB:Finch_MAW:Roberts_SM:Woolley_GT:Cave_RJ:and Newton_RF:,J. Chem. Soc.,1980,,2093| | ||
|Mass Spectra=TMS ETHER ; M | {{RelationTable/GetFirstAuthor|Reference:Mubarik-Ali_S:Chapeleo_CB:Finch_MAW:Roberts_SM:Woolley_GT:Cave_RJ:and Newton_RF:,J. Chem. Soc.,1980,,2093}}]], CHLOROFORM, ETHANOL [[Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790}}]] | ||
|UV Spectra= | |Mass Spectra=TMS ETHER ; M^+ 478.2934 [[Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790}}]] | ||
|IR Spectra= | |UV Spectra= lambda ^{MeOH}_{max} = 305( epsilon 1200), 216( epsilon 9900)nm [[Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790}}]] | ||
|NMR Spectra= | |IR Spectra= nu 3400, 3200, 2660, 1710, 1085, 970 cm^{-1} [[Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790}}]] | ||
|NMR Spectra=^1 H-NMR(CDCl_3 ) : delta 7.75-7.55(m, 1H, 9-CH), 6.30-6.10(m, 1H, 10-CH), 5.90(brs, 2H, OH), 5.75-5.35(m, 4H), 4.30-3.95(m, 1H, 15-CH) [[Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790}}]] | |||
|Source= | |||
|Chemical Synthesis=[[Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790}}]] {{Image200|LBF20307PG38FT0001.gif}} | |||
|Metabolism=In aqueous solution prostaglandin D2 undergoes non-enzymatic dehydration and is converted to prostaglandin J2 [[Reference:Fukushima_M:,Prostaglandins Leukot. Essent. Fatty Acids,1992,47,1|{{RelationTable/GetFirstAuthor|Reference:Fukushima_M:,Prostaglandins Leukot. Essent. Fatty Acids,1992,47,1}}]]. | |||
|Symbol=PGJ2 | |||
|Biological Activity=The anti-tumor and anti-viral activities of prostaglandin J2 are attributed to Delta 12-prostaglandin J2 which is a degradation product of prostagladnin J2 and is characteristic of its alkylidene cyclopentenone structure [[Reference:Fukushima_M:,Prostaglandins Leukot. Essent. Fatty Acids,1992,47,1|{{RelationTable/GetFirstAuthor|Reference:Fukushima_M:,Prostaglandins Leukot. Essent. Fatty Acids,1992,47,1}}]]. | |||
}} | |||
{{MassbankSpectra| | |||
UT000361 | |||
UT000362 | |||
UT000363 | |||
UT000364 | |||
UT000365 | |||
UT000366 | |||
UT000367 | |||
UT000368 | |||
UT000369 | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 09:00, 17 January 2014
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1901 |
| LipidMaps | LMFA03010019 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20307PG38 |
| Prostaglandin J2 | |
|---|---|
| |
| Structural Information | |
| 7- [2R- (3S-hydroxy-trans-1-octenyl) -3-oxo-4-cyclopenten-1R-yl] -cis-5-heptenoic acid | |
| |
| PGJ2 | |
| Formula | C20H30O4 |
| Exact Mass | 334.21440944799997 |
| Average Mass | 334.4498 |
| SMILES | C(CC[C@@H](O)C=C[C@@H](C(=O)1)[C@@H](CC=CCCCC(O)=O)C=C1)CC |
| Physicochemical Information | |
| ACETONITRILE Mubarik-AliSet al., CHLOROFORM, ETHANOL Bundy_GL et al. | |
Bundy_GL et al.  | |
| In aqueous solution prostaglandin D2 undergoes non-enzymatic dehydration and is converted to prostaglandin J2 Fukushima_M . | |
| The anti-tumor and anti-viral activities of prostaglandin J2 are attributed to Delta 12-prostaglandin J2 which is a degradation product of prostagladnin J2 and is characteristic of its alkylidene cyclopentenone structure Fukushima_M . | |
| Spectral Information | |
| Mass Spectra | TMS ETHER ; M+ 478.2934 Bundy_GL et al. |
| UV Spectra | λ MeOH max = 305( ε 1200), 216( ε 9900)nm Bundy_GL et al. |
| IR Spectra | ν 3400, 3200, 2660, 1710, 1085, 970 cm-1 Bundy_GL et al. |
| NMR Spectra | 1H-NMR(CDCl3) : δ 7.75-7.55(m, 1H, 9-CH), 6.30-6.10(m, 1H, 10-CH), 5.90(brs, 2H, OH), 5.75-5.35(m, 4H), 4.30-3.95(m, 1H, 15-CH) Bundy_GL et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|








