LBF20306EO03: Difference between revisions
No edit summary |
No edit summary |
||
| (18 intermediate revisions by 2 users not shown) | |||
| Line 4: | Line 4: | ||
{{Metabolite | {{Metabolite | ||
|LipidBank=DFA8112 | |LipidBank=DFA8112, XPR6302 | ||
|LipidMaps=- | |LipidMaps=LMFA03080004 | ||
|SysName= | |CAS=81276-02-0 | ||
|Common Name=&&(+-) 11 (12) - | |SysName=dl-11 (12) -Epoxy- (cis-5,cis-8,cis-14) -eicosatrienoic acid | ||
|Source=(±)11(12)-EpETrE is biosynthesized in rat and rabbit liver microsomes by cytochrome P450 [[Reference:Oliw_EH:Guengerich_FP:Oates_JA:,J. Biol. Chem.,1982,257,3771|{{RelationTable/GetFirstAuthor|Reference:Oliw_EH:Guengerich_FP:Oates_JA:,J. Biol. Chem.,1982,257,3771}}]][[Reference:Chacos_N:Falck_JR:Wixtrom_C:Capdevila_J:,Biochem. Biophys. Res. Commun.,1982,104,916|{{RelationTable/GetFirstAuthor|Reference:Chacos_N:Falck_JR:Wixtrom_C:Capdevila_J:,Biochem. Biophys. Res. Commun.,1982,104,916}}]]. | |Common Name=&&(+-) -11 (12) -Epoxy- (5Z,8Z,14Z) -eicosatrienoic acid&&11,12-EET&& | ||
|Chemical Synthesis= | |Optical=11(R),12(S)-EET METHYL ESTER ; [ alpha ]^{23}_D=+4.94°(C=1.64, ACETONE) [[Reference:Mosset_P:Yadagiri_P:Lumin_S:Capdevila_J:Falck_JR:,Tetrahedron_Lett.,1986,27,6035|{{RelationTable/GetFirstAuthor|Reference:Mosset_P:Yadagiri_P:Lumin_S:Capdevila_J:Falck_JR:,Tetrahedron_Lett.,1986,27,6035}}]]<!--1098-->, 11(S),12(R)-EET METHYL ESTER ; [ alpha ]^{24}_D=-2.34°(C=0.66, CHLOROFORM) [[Reference:Falck_JR:Manna_S:Capdevila_J:,Tetrahedron_Lett.,1984,25,2443|{{RelationTable/GetFirstAuthor|Reference:Falck_JR:Manna_S:Capdevila_J:,Tetrahedron_Lett.,1984,25,2443}}]]<!--1099--> | ||
|NMR Spectra=^1 H-NMR(CDCl3) : delta 5.54-5.20(m, 6H), 3.61(s, 3H), 3.00-2.68(m, 4H), 2.37-2.00(m, 10H), 1.81-1.54(m, 2H), 1.46-1.13(m, 6H), 0.88(t, J=7Hz, 3H) [[Reference:Mosset_P:Yadagiri_P:Lumin_S:Capdevila_J:Falck_JR:,Tetrahedron_Lett.,1986,27,6035|{{RelationTable/GetFirstAuthor|Reference:Mosset_P:Yadagiri_P:Lumin_S:Capdevila_J:Falck_JR:,Tetrahedron_Lett.,1986,27,6035}}]]<!--1098--> | |||
|Mass Spectra=METHYL ESTER ; m/e 340(M^+ ), 322, 309, 227, 155 [[Reference:Oliw_EH:Guengerich_FP:Oates_JA:,J._Biol._Chem.,1982,257,3771|{{RelationTable/GetFirstAuthor|Reference:Oliw_EH:Guengerich_FP:Oates_JA:,J._Biol._Chem.,1982,257,3771}}]]<!--1096-->. | |||
|Source=(±)11(12)-EpETrE is biosynthesized in rat and rabbit liver microsomes by cytochrome P450 [[Reference:Oliw_EH:Guengerich_FP:Oates_JA:,J. Biol. Chem.,1982,257,3771|{{RelationTable/GetFirstAuthor|Reference:Oliw_EH:Guengerich_FP:Oates_JA:,J. Biol. Chem.,1982,257,3771}}]][[Reference:Chacos_N:Falck_JR:Wixtrom_C:Capdevila_J:,Biochem. Biophys. Res. Commun.,1982,104,916|{{RelationTable/GetFirstAuthor|Reference:Chacos_N:Falck_JR:Wixtrom_C:Capdevila_J:,Biochem. Biophys. Res. Commun.,1982,104,916}}]]. The compound is produced when arachidonic acid in the presence of NADPH is incubated with the liver microsome of rat [[Reference:Chacos_N:Falck_JR:Wixtrom_C:Capdevila_J:,Biochem._Biophys._Res._Commun.,1982,104,916|{{RelationTable/GetFirstAuthor|Reference:Chacos_N:Falck_JR:Wixtrom_C:Capdevila_J:,Biochem._Biophys._Res._Commun.,1982,104,916}}]]<!--0076--> or rabbit [[Reference:Oliw_EH:Guengerich_FP:Oates_JA:,J._Biol._Chem.,1982,257,3771|{{RelationTable/GetFirstAuthor|Reference:Oliw_EH:Guengerich_FP:Oates_JA:,J._Biol._Chem.,1982,257,3771}}]]<!--0077--> | |||
|Chemical Synthesis=[http://lipidbank.jp/image/XPR6302FT0001.gif Table0001] [[Reference:Mosset_P:Yadagiri_P:Lumin_S:Capdevila_J:Falck_JR:,Tetrahedron_Lett.,1986,27,6035|{{RelationTable/GetFirstAuthor|Reference:Mosset_P:Yadagiri_P:Lumin_S:Capdevila_J:Falck_JR:,Tetrahedron_Lett.,1986,27,6035}}]]<!--1098--> | |||
|Metabolism= | |Metabolism= | ||
|Symbol=(+-)11(12)-EpETrE | |||
|Biological Activity=(±)11(12)-EpETrE has been shown, along with (±)8(9)-EpETrE, to play a role in the recovery of depleted Ca^{2+} pools in cultured smooth muscle cells [[Reference:Graber_MN:Alfonso_A:Gill_DL:,J. Biol. Chem.,1997,272,29546|{{RelationTable/GetFirstAuthor|Reference:Graber_MN:Alfonso_A:Gill_DL:,J. Biol. Chem.,1997,272,29546}}]]. The compound relaxes intestinal artery, inhibits vasopressin-dependent water flow in urinary bladder, and regulates intracellular calcium level [[Reference:Fitzpatrick_FA:Murphy_RC:,Pharmacol._Rev.,1988,40,229|{{RelationTable/GetFirstAuthor|Reference:Fitzpatrick_FA:Murphy_RC:,Pharmacol._Rev.,1988,40,229}}]]<!--0078-->. | |||
}} | |||
{{MassbankSpectra| | |||
UT000001 | |||
UT000002 | |||
UT000003 | |||
UT000004 | |||
UT000005 | |||
UT000006 | |||
UT000007 | |||
UT000008 | |||
UT000009 | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 09:00, 21 January 2014
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR6302 DFA8112, XPR6302 |
| LipidMaps | LMFA03080004 |
| CAS | 81276-02-0 |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20306EO03 |
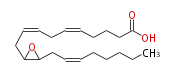
| (+-) -11 (12) -Epoxy- (5Z,8Z,14Z) -eicosatrienoic acid | |
|---|---|
| |
| Structural Information | |
| dl-11 (12) -Epoxy- (cis-5,cis-8,cis-14) -eicosatrienoic acid | |
| |
| (+-)11(12)-EpETrE | |
| Formula | C20H32O3 |
| Exact Mass | 320.23514489 |
| Average Mass | 320.46628 |
| SMILES | C(CC=CCC(O1)C1CC=CCC=CCCCC(O)=O)CCC |
| Physicochemical Information | |
| 11(R),12(S)-EET METHYL ESTER ; [ α ]23 D =+4.94°(C=1.64, ACETONE) MossetPet al., 11(S),12(R)-EET METHYL ESTER ; [ α ]24 D =-2.34°(C=0.66, CHLOROFORM) Falck_JR et al. | |
| (±)11(12)-EpETrE is biosynthesized in rat and rabbit liver microsomes by cytochrome P450 Oliw_EH et al. Chacos_N et al.. The compound is produced when arachidonic acid in the presence of NADPH is incubated with the liver microsome of rat Chacos_N et al. or rabbit Oliw_EH et al. | |
| Table0001 Mosset_P et al. | |
| (±)11(12)-EpETrE has been shown, along with (±)8(9)-EpETrE, to play a role in the recovery of depleted Ca^{2+} pools in cultured smooth muscle cells Graber_MN et al.. The compound relaxes intestinal artery, inhibits vasopressin-dependent water flow in urinary bladder, and regulates intracellular calcium level Fitzpatrick_FA et al.. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER ; m/e 340(M+), 322, 309, 227, 155 Oliw_EH et al.. |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 1H-NMR(CDCl3) : δ 5.54-5.20(m, 6H), 3.61(s, 3H), 3.00-2.68(m, 4H), 2.37-2.00(m, 10H), 1.81-1.54(m, 2H), 1.46-1.13(m, 6H), 0.88(t, J=7Hz, 3H) MossetPet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|








