LBF13101SC01: Difference between revisions
No edit summary |
No edit summary |
||
| (11 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 8: | Line 10: | ||
|Melting Point=38-39°C | |Melting Point=38-39°C | ||
|Boiling Point=192°C at 20 mmHg | |Boiling Point=192°C at 20 mmHg | ||
|Solubility=[[Reference:Ahmad_K:Bumpus_FM:Strong_FM:,J. Am. Chem. Soc.,1948,70,3391|{{RelationTable/GetFirstAuthor|Reference:Ahmad_K:Bumpus_FM:Strong_FM:,J. Am. Chem. Soc.,1948,70,3391}}]][[Reference:Chuit_P:Boelsing_F:Hausser_J:Malet_G:,Helv. Chim. Acta,1927,10,113|{{RelationTable/GetFirstAuthor|Reference:Chuit_P:Boelsing_F:Hausser_J:Malet_G:,Helv. Chim. Acta,1927,10,113}}]] | |Solubility=[[Reference:Ahmad_K:Bumpus_FM:Strong_FM:,J. Am. Chem. Soc.,1948,70,3391|{{RelationTable/GetFirstAuthor|Reference:Ahmad_K:Bumpus_FM:Strong_FM:,J. Am. Chem. Soc.,1948,70,3391}}]]<!--0017--><!--0069-->[[Reference:Chuit_P:Boelsing_F:Hausser_J:Malet_G:,Helv. Chim. Acta,1927,10,113|{{RelationTable/GetFirstAuthor|Reference:Chuit_P:Boelsing_F:Hausser_J:Malet_G:,Helv. Chim. Acta,1927,10,113}}]] | ||
|Source= | |||
|Chemical Synthesis=Synthetic by (i) reaction of 1-bromo-10-undecene ethyl malonate and Na and (ii) conversion of 10-undecenoic acid to 11-dodecenoic acid. | |||
|Metabolism= | |||
|Symbol=C13:1 | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 06:00, 15 April 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA0085 |
| LipidMaps | LMFA01030046 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF13101SC01 |
| 12-Tridecenoic acid | |
|---|---|
| |
| Structural Information | |
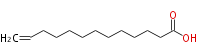
| 12-Tridecenoic acid | |
| |
| C13:1 | |
| Formula | C13H24O2 |
| Exact Mass | 212.17763001199998 |
| Average Mass | 212.32845999999998 |
| SMILES | C=CCCCCCCCCCCC(O)=O |
| Physicochemical Information | |
| 38-39°C | |
| 192°C at 20 mmHg | |
| AhmadKet al. ChuitPet al. | |
| Synthetic by (i) reaction of 1-bromo-10-undecene ethyl malonate and Na and (ii) conversion of 10-undecenoic acid to 11-dodecenoic acid. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|