LBF17307HO02: Difference between revisions
No edit summary |
No edit summary |
||
| (8 intermediate revisions by 2 users not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=XPR6201 | |LipidBank=XPR6201 | ||
|LipidMaps=- | |LipidMaps=- | ||
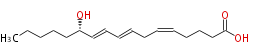
|SysName= | |SysName= 12S-Hydroxy- (cis-5,trans-8,trans-10) -heptadecatrienoic acid | ||
|Common Name=&&(S) | |Common Name=&&12S-Hydroxy- (5Z,8E,10E) -heptadecatrienoic acid&&(S)- (Z,E,E) -12-Hydroxy-5,8,10-heptadecatrienoic acid&& | ||
| | |Optical=METHYL ESTER ; [ alpha ]^{25}_D =+7.5°(C=0.2, CHLOROFORM) [[Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131|{{RelationTable/GetFirstAuthor|Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131}}]] | ||
|Solubility=DIETHYL ETHER [[Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400}}]] | |Solubility=DIETHYL ETHER [[Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400}}]] | ||
|Mass Spectra=METHYL ESTER TMS ETHER ; m/e 366(M | |Mass Spectra=METHYL ESTER TMS ETHER ; m/e 366(M^+ ), 335, 295, 276, 225, 173, (128)[[Reference:Hamberg_M:Svensson_J:Wakabayashi_T:Samuelsson_B:,Proc. Nat. Acad. Sci. USA,1974,71,345|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Svensson_J:Wakabayashi_T:Samuelsson_B:,Proc. Nat. Acad. Sci. USA,1974,71,345}}]] METHYL ESTER ; 298(M^+ ), 224 [[Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131|{{RelationTable/GetFirstAuthor|Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131}}]] | ||
|UV Spectra=METHYL ESTER ; ETHANOL : 232nm( | |UV Spectra=METHYL ESTER ; ETHANOL : 232nm( epsilon 33,400)[[Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400}}]]. METHANOL : 240nm [[Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131|{{RelationTable/GetFirstAuthor|Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131}}]] | ||
|NMR Spectra=METHYL ESTER ; | |NMR Spectra=METHYL ESTER ; ^1 H-NMR(CDCl_3 ) : delta 6.17(dd, J=15.11, 10.36Hz, 1H, 10-CH), 6.04(dd, J=15,05, 10.52Hz, 1H, 9-CH), 5.66(dt, J=15.16, 6.48Hz, 1H), 5.60(dd, J=17.17, 7.04Hz, 1H, 11-CH), 5.42(m, 2H, 5-CH, 6-CH), 4.1(m, 1H, 12-CH), 3.66(s, 3H, COOCH_3 ), 2.81(m, 2H, 7-CH), 2.36(t, J=7.51Hz, 2H, 2-CH), 2.1-0.85(m, 16H, CH_2 and CH_3 ) [[Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131|{{RelationTable/GetFirstAuthor|Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131}}]] | ||
|Source= | |Source= | ||
|Chemical Synthesis=[[Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131|{{RelationTable/GetFirstAuthor|Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131}}]] {{Image200|LBF17307HO02FT0001.gif}} | |Chemical Synthesis=[[Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131|{{RelationTable/GetFirstAuthor|Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131}}]] {{Image200|LBF17307HO02FT0001.gif}} | ||
|Metabolism=When prostaglandin H2 reacts with thromboxane A synthase and the endoperoxide moiety is cleaved, the production of thromboxane A2 is accompanied by the formation of 12(S)-hydroxy-5,8,10-heptadecatrienoic acid in an almost equimolar amount liberating malondialdehyde [[Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400}}]]. This compound is also a product of non-enxymatic degradation of prostaglandin H2 [[Reference:Nugteren_DH:Hazelhof_E:,Biochim. Biophys. Acta,1973,326,448|{{RelationTable/GetFirstAuthor|Reference:Nugteren_DH:Hazelhof_E:,Biochim. Biophys. Acta,1973,326,448}}]]. | |Metabolism=When prostaglandin H2 reacts with thromboxane A synthase and the endoperoxide moiety is cleaved, the production of thromboxane A2 is accompanied by the formation of 12(S)-hydroxy-5,8,10-heptadecatrienoic acid in an almost equimolar amount liberating malondialdehyde [[Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400}}]]. This compound is also a product of non-enxymatic degradation of prostaglandin H2 [[Reference:Nugteren_DH:Hazelhof_E:,Biochim. Biophys. Acta,1973,326,448|{{RelationTable/GetFirstAuthor|Reference:Nugteren_DH:Hazelhof_E:,Biochim. Biophys. Acta,1973,326,448}}]]. | ||
|Symbol=HHT | |||
|Biological Activity=The compound stimulates chemotactic and chemokinetic activities of human polymorphonuclear leukocytes [[Reference:Goetzl_EJ:Gorman_RR:,J. Immunol.,1978,120,526|{{RelationTable/GetFirstAuthor|Reference:Goetzl_EJ:Gorman_RR:,J. Immunol.,1978,120,526}}]]. | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 15:24, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR6201 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF17307HO02 |
| 12S-Hydroxy- (5Z,8E,10E) -heptadecatrienoic acid | |
|---|---|
| |
| Structural Information | |
| 12S-Hydroxy- (cis-5,trans-8,trans-10) -heptadecatrienoic acid | |
| |
| HHT | |
| Formula | C17H28O3 |
| Exact Mass | 280.203844762 |
| Average Mass | 280.40242 |
| SMILES | CCCCC[C@H](O)C=CC=CCC=CCCCC(O)=O |
| Physicochemical Information | |
| METHYL ESTER ; [ α ]25 D =+7.5°(C=0.2, CHLOROFORM) Nicolaou_KC et al. | |
| DIETHYL ETHER HambergMet al. | |
Nicolaou_KC et al.  | |
| When prostaglandin H2 reacts with thromboxane A synthase and the endoperoxide moiety is cleaved, the production of thromboxane A2 is accompanied by the formation of 12(S)-hydroxy-5,8,10-heptadecatrienoic acid in an almost equimolar amount liberating malondialdehyde Hamberg_M et al.. This compound is also a product of non-enxymatic degradation of prostaglandin H2 Nugteren_DH et al.. | |
| The compound stimulates chemotactic and chemokinetic activities of human polymorphonuclear leukocytes Goetzl_EJ et al.. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER TMS ETHER ; m/e 366(M+), 335, 295, 276, 225, 173, (128) HambergMet al. METHYL ESTER ; 298(M+), 224 Nicolaou_KC et al. |
| UV Spectra | METHYL ESTER ; ETHANOL : 232nm( ε 33,400) HambergMet al.. METHANOL : 240nm Nicolaou_KC et al. |
| IR Spectra | |
| NMR Spectra | METHYL ESTER ; 1H-NMR(CDCl3) : δ 6.17(dd, J=15.11, 10.36Hz, 1H, 10-CH), 6.04(dd, J=15,05, 10.52Hz, 1H, 9-CH), 5.66(dt, J=15.16, 6.48Hz, 1H), 5.60(dd, J=17.17, 7.04Hz, 1H, 11-CH), 5.42(m, 2H, 5-CH, 6-CH), 4.1(m, 1H, 12-CH), 3.66(s, 3H, COOCH3), 2.81(m, 2H, 7-CH), 2.36(t, J=7.51Hz, 2H, 2-CH), 2.1-0.85(m, 16H, CH2 and CH3) Nicolaou_KC et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|