LBF18109EO01: Difference between revisions
No edit summary |
No edit summary |
||
| (16 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
{{Metabolite | {{Metabolite | ||
|LipidBank=DFA8008 | |LipidBank=DFA8008 | ||
|LipidMaps= | |LipidMaps=LMFA02000038 | ||
|SysName=12,13-Epoxy-9- | |SysName=12,13-Epoxy-9-octadecenoic acid | ||
|Common Name=&&12,13- | |Common Name=&&12,13-EODE&& | ||
|Mass Spectra=GC-EI-MS(after solvolysation-trimethylsilylation in MeOH)[[Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31|{{RelationTable/GetFirstAuthor|Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31}}]][[Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971|{{RelationTable/GetFirstAuthor|Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]]: m/e= 299[SNTO=CH-CH2-CH=CH(CH2)7COOCH3], 217[CH3(CH2)4CH(OCH3)CHOTMS], 195[OHCCH2CH=CH(CH2)7CO], 173[ SMTO=CH(CH2)4CH3] | |Mass Spectra=GC-EI-MS(after solvolysation-trimethylsilylation in MeOH)[[Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31|{{RelationTable/GetFirstAuthor|Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31}}]][[Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971|{{RelationTable/GetFirstAuthor|Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]]: m/e= 299[SNTO=CH-CH2-CH=CH(CH2)7COOCH3], 217[CH3(CH2)4CH(OCH3)CHOTMS], 195[OHCCH2CH=CH(CH2)7CO], 173[ SMTO=CH(CH2)4CH3] | ||
|IR Spectra=Trans olefin(960cm | |IR Spectra=Trans olefin(960cm^{-1}), cis olefin(720cm^{-1}), trans epoxide(885cm^{-1}), cis epoxide(840 and 820cm^{-1})[[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]] | ||
|NMR Spectra= | |NMR Spectra=^1 H-NMR[[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]]: C8(2.01ppm), C9, 10(5.45ppm), C2, 11(2.29ppm), C12, 13(2.91ppm), J9-10= 10Hz(cis olefin) | ||
|Source=A major secondary product by autooxidation in a lipid film of linoleate[[Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971|{{RelationTable/GetFirstAuthor|Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971}}]]. A bitter substance in lecithin[[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]]. | |||
|Chemical Synthesis= | |||
|Metabolism= | |||
|Biological Activity=Antimicrobial compounds for Piricularia oryzae, a pathogenic fungus of rice blast disease (Imochi-byo)[[Reference:Kato_T:Yamaguthi_H:Uehara_T:Namai_T:,Chemistry and Biology (in Japanese),1986,24,183|{{RelationTable/GetFirstAuthor|Reference:Kato_T:Yamaguthi_H:Uehara_T:Namai_T:,Chemistry and Biology (in Japanese),1986,24,183}}]] | |||
}} | |||
{{MassbankSpectra| | |||
UT000010 | |||
UT000011 | |||
UT000012 | |||
UT000013 | |||
UT000014 | |||
UT000015 | |||
UT000016 | |||
UT000017 | |||
UT000018 | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 09:00, 17 January 2014
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8008 |
| LipidMaps | LMFA02000038 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18109EO01 |
| 12,13-EODE | |
|---|---|
| |
| Structural Information | |
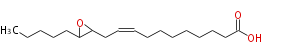
| 12,13-Epoxy-9-octadecenoic acid | |
| |
| Formula | C18H32O3 |
| Exact Mass | 296.23514489 |
| Average Mass | 296.44488 |
| SMILES | C(C(CC=CCCCCCCCC(O)=O)1)(CCCCC)O1 |
| Physicochemical Information | |
| A major secondary product by autooxidation in a lipid film of linoleate Wu_GS et al.. A bitter substance in lecithin Sessa_DJ et al.. | |
| Antimicrobial compounds for Piricularia oryzae, a pathogenic fungus of rice blast disease (Imochi-byo) Kato_T et al. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after solvolysation-trimethylsilylation in MeOH) KleimanRet al. Wu_GS et al. Sessa_DJ et al.: m/e= 299[SNTO=CH-CH2-CH=CH(CH2)7COOCH3], 217[CH3(CH2)4CH(OCH3)CHOTMS], 195[OHCCH2CH=CH(CH2)7CO], 173[ SMTO=CH(CH2)4CH3] |
| UV Spectra | |
| IR Spectra | Trans olefin(960cm-1), cis olefin(720cm-1), trans epoxide(885cm-1), cis epoxide(840 and 820cm-1) Sessa_DJ et al. |
| NMR Spectra | 1H-NMR Sessa_DJ et al.: C8(2.01ppm), C9, 10(5.45ppm), C2, 11(2.29ppm), C12, 13(2.91ppm), J9-10= 10Hz(cis olefin) |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|