LBF18206EO01: Difference between revisions
No edit summary |
No edit summary |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=DFA8073 | |LipidBank=DFA8073 | ||
|LipidMaps=LMFA01070014 | |LipidMaps=LMFA01070014 | ||
|SysName=Methyl-15,16- | |SysName=Methyl-15,16-epoxy-9,12-octadecadienoic acid | ||
|Common Name=&&Methyl-15,16- | |Common Name=&&Methyl-15,16-epoxy-9,12-octadecadienoate&& | ||
|Mass Spectra=GC-EI-MS[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780}}]]: m/e=308[M]; 279[CH-(O)-CHCH2CH=CHCH2CH=CH(CH2)7COOCH3]; 277[M -OCH3]; 251[279-28]; 71[CH3CH2CH-(O)-CH]; 43[71-28]; GC-EI-MS(after hydrogenation)(105): m/e=281[M-OCH3]; 255[CH-(O)-CH(CH2)13COOCH3-28]; 71[CH3CH2CH-(O)-CH]; 43[71-28] | |Mass Spectra=GC-EI-MS[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780}}]]: m/e=308[M]; 279[CH-(O)-CHCH2CH=CHCH2CH=CH(CH2)7COOCH3]; 277[M -OCH3]; 251[279-28]; 71[CH3CH2CH-(O)-CH]; 43[71-28]; GC-EI-MS(after hydrogenation)(105): m/e=281[M-OCH3]; 255[CH-(O)-CH(CH2)13COOCH3-28]; 71[CH3CH2CH-(O)-CH]; 43[71-28] | ||
|IR Spectra=Cis unsaturation: 3002cm | |IR Spectra=Cis unsaturation: 3002cm^{-1}[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439}}]] | ||
|NMR Spectra= | |NMR Spectra=^1 H-NMR[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439}}]]: cis unsaturationS: 5.43ppm[4H]; cis epoxide ring:2.79 AND 2.98ppm[2H] | ||
|Source=Auto oxidation of linoleate[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439}}]] | |Source=Auto oxidation of linoleate[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439}}]]. Photoenhancement of linoleate peroxidation[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780}}]]. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
Latest revision as of 06:00, 15 April 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8073 |
| LipidMaps | LMFA01070014 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18206EO01 |
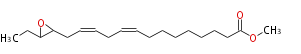
| Methyl-15,16-epoxy-9,12-octadecadienoate | |
|---|---|
| |
| Structural Information | |
| Methyl-15,16-epoxy-9,12-octadecadienoic acid | |
| |
| Formula | C19H32O3 |
| Exact Mass | 308.23514489 |
| Average Mass | 308.45558 |
| SMILES | C(CC=CCC=CCCCCCCCC(=O)OC)(O1)C(CC)1 |
| Physicochemical Information | |
| Auto oxidation of linoleate Neff_WE et al.. Photoenhancement of linoleate peroxidation Neff_WE et al.. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS Neff_WE et al.: m/e=308[M]; 279[CH-(O)-CHCH2CH=CHCH2CH=CH(CH2)7COOCH3]; 277[M -OCH3]; 251[279-28]; 71[CH3CH2CH-(O)-CH]; 43[71-28]; GC-EI-MS(after hydrogenation)(105): m/e=281[M-OCH3]; 255[CH-(O)-CH(CH2)13COOCH3-28]; 71[CH3CH2CH-(O)-CH]; 43[71-28] |
| UV Spectra | |
| IR Spectra | Cis unsaturation: 3002cm-1 Neff_WE et al. |
| NMR Spectra | 1H-NMR Neff_WE et al.: cis unsaturationS: 5.43ppm[4H]; cis epoxide ring:2.79 AND 2.98ppm[2H] |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|