LBF18305SC03: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|LipidBank=DFA0187 | |LipidBank=DFA0187 | ||
|LipidMaps=LMFA01030148 | |LipidMaps=LMFA01030148 | ||
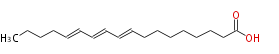
|SysName=trans-9, trans-11, trans-13-Octadecatrienoic acid | |SysName=(trans-9,trans-11,trans-13) -Octadecatrienoic acid | ||
|Common Name=&&(9E,11E,13E) -Octadecatrienoic acid&&beta-Eleostearic acid&& | |Common Name=&&(9E,11E,13E) -Octadecatrienoic acid&&beta-Eleostearic acid&& | ||
|Melting Point=61.5-62.5°C | |Melting Point=61.5-62.5°C | ||
Latest revision as of 14:01, 5 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA0187 |
| LipidMaps | LMFA01030148 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18305SC03 |
| (9E,11E,13E) -Octadecatrienoic acid | |
|---|---|
| |
| Structural Information | |
| (trans-9,trans-11,trans-13) -Octadecatrienoic acid | |
| |
| C18:3 | |
| Formula | C18H30O2 |
| Exact Mass | 278.224580204 |
| Average Mass | 278.4296 |
| SMILES | CCCCC=CC=CC=CCCCCCCCC(O)=O |
| Physicochemical Information | |
| 61.5-62.5°C | |
| soluble in acetone, ethanol, CS2, heptane, methyl alcohol and pentane. | |
| Cattle-liver phosphatides. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
There are 8 possible isomers, and 4 are naturally identified. |
3つある二重結合の構造異性体は8種類考えられるが、4種が天然に見つかっている。 |
- α-eleostearic acid (cis, trans, trans) LBF18305SC02 m.p. 48 °C
The most common acid of this group. Principal acid of tung oil or China Wood oil, and the seed oil of Momordica charantia and Centranthus macrosiphon. It is easily converted to β-eleostearic acid by light, heat, sulfur, acid, or iodine. |
もっとも一般的で、ツング(中国では四川省に生える油桐)油、 Momordica charantia や Centranthus macrosiphon の種から取れる油の主成分。光や熱、硫黄、酸、ヨウ素などによって容易に β-eleostearic acid に変化する。 |
- Punicic acid (cis, trans, cis) LBF18305SC01 m.p. 41 °C
Principal acid of pomegranate seed oil. Yellow. It is easily coverted to β-eleostearic acid by the same catalysts. Its alternative name is Trichosanic acid, a major component of Trichosanthes kirilowii japonica seed oil. It shows platelet aggregation [1]
The result contradicts other results (Punicic acid is major, e.g. [3]), and is considered an error. (By:Masanori Arita) |
ザクロ の種から取れる黄色い油の主成分。ザクロの属名 Punica より名がついている。 α-eleostearic acidと同様に、同じ触媒によって簡単に β-eleostearic acid に変化する。別名は Trichosanic acid で、日本のでヘビウリと呼ばれる Trichosanthes kirilowii japonica 種子の主成分。血小板の凝固作用がある。
|
- β-eleostearic acid (trans, trans, trans) LBF18305SC03 m.p. 72 °C
Transformed form of α-eleostearic acid or punicic acid. |
α-eleostearic acid や punicic acid が変化したもの。 |
- Catalpic acid (trans, trans, cis) m.p. 32 °C
Principal acid of Catalpa ovata, Catalpa speciosa and Catalpa bignonioides seed oil. |
Catalpa ovata, Catalpa speciosa や Catalpa bignonioides 種子油の主成分。 |
- References
- ↑ Takenaga M, Hirai A, Terano T, Tamura Y, Kitagawa H, Yoshida S (1988) "In vitro effect of trichosanic acid, a major component of Trichosanthes japonica on platelet aggregation and arachidonic acid metabolism in human platelets" Prostaglandins Leukot Essent Fatty Acids 31(2):65-72
- ↑ Fatope MO, Al Burtomani SK, Takeda Y (2002) "Monoacylglycerol from Punica granatum Seed Oil" J Agric Food Chem 50:357-360
- ↑ Kyralan M, Golukcu M, Tokgoz H (2009) "Oil and Conjugated Linolenic Acid Contents of Seeds from Important Pomegranate Cultivars (Punica granatum L.) Grown in Turkey" J Am Oil Chem Soc 86:985–990