LBF20207PG27: Difference between revisions
No edit summary |
No edit summary |
||
| (17 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=XPR1601 | |LipidBank=XPR1601 | ||
|LipidMaps=LMFA03010009 | |LipidMaps=LMFA03010009 | ||
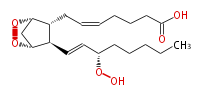
|SysName=7- [ | |SysName=7- [2R- (3S -Hydroxy-trans-1-octenyl) -(1R,4S) -5,6-dioxabicyclo [ 2.2.1 ] -heptan-3R-yl] -cis-5-heptenoic acid | ||
|Common Name=&& | |Common Name=&&Prostaglandin G_2&&7- [ 2R- (3S -Hydroxy-1-(E)-octenyl) -(1R,4S) -5,6-dioxabicyclo [ 2.2.1 ] -heptan-3R-yl ] -5(Z)-heptenoic acid&& | ||
|NMR Spectra= | |NMR Spectra=^{13}C-NMR : delta 84.6(C15) [[Reference:Porter_NA:Byers_JD:Ali_AE:Eling_TE:,J. Am. Chem. Soc.,1980,102,1183|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Byers_JD:Ali_AE:Eling_TE:,J. Am. Chem. Soc.,1980,102,1183}}]] | ||
|Source=Prostaglandin G2 is produced by bis-dioxygenation and cyclization of arachidonic acid as an intermediate for the biosyntheses of various prostaglandins and thromboxanes. The responsible enzyme is prostaglandin endoperoxide synthase usually referred to as fatty acid cyclooxygenase, and distributed in various animal tissues [[Reference:Pace-Asciak_CR:Smith_WL:,The_Enzymes,1983,16,543|{{RelationTable/GetFirstAuthor|Reference:Pace-Asciak_CR:Smith_WL:,The_Enzymes,1983,16,543}}]] <!--0019-->. | |||
|Chemical Synthesis=[[Reference:Porter_NA:Byers_JD:Ali_AE:Eling_TE:,J. Am. Chem. Soc.,1980,102,1183|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Byers_JD:Ali_AE:Eling_TE:,J. Am. Chem. Soc.,1980,102,1183}}]] {{Image200|LBF20207PG27FT0001.gif}} | |||
|Metabolism=Prostaglandin endoperoxide synthase is a bifunctional enzyme with an oxygenase activity (fatty acid cyclooxygenase) producing prostaglandin G2 from arachidonic acid and a peroxidase activity (prostagladnin hydroperoxidase) converting prostaglandin G2 to prostaglandin H2 <!--0019-->. The enzyme is usually referred to briefly as cyclooxygenase. There are two isozymes of the enzyme. Cyclooxygenase-1 is a constitutive enzyme expressed in most mammalian cells, while cyclooxygenase-2 is a product of immediate early gene and is induced rapidly and transiently in certain types of cell by various bioactive compounds [[Reference:Smith_WL:Garavito_RM:DeWitt_DL:,J. Biol. Chem.,1996,271,33157|{{RelationTable/GetFirstAuthor|Reference:Smith_WL:Garavito_RM:DeWitt_DL:,J. Biol. Chem.,1996,271,33157}}]][[Reference:Herschman_HR:,Biochim._Biophys._Acta,1996,1299,125|{{RelationTable/GetFirstAuthor|Reference:Herschman_HR:,Biochim._Biophys._Acta,1996,1299,125}}]] | |||
|Symbol=PGG2 | |||
|Biological Activity=Prostaglandin G2 is a metabolic intermediate, but the compound as such has biological activities of bronchoconstriction and vasoconstriction [[Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293|{{RelationTable/GetFirstAuthor|Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293}}]][[Reference:Chijimatsu_Y:Nguyen_TV:Said_SI:,Prostaglandins,1977,13,909|{{RelationTable/GetFirstAuthor|Reference:Chijimatsu_Y:Nguyen_TV:Said_SI:,Prostaglandins,1977,13,909}}]]. | |||
|Genetic Information=cDNA and genomic DNA each for cyclooxygenas-1 and 2 were cloned [[Reference:Funk_CD:,Prog Nucleic Acid Res Mol Biol.,1993,45,67|{{RelationTable/GetFirstAuthor|Reference:Funk_CD:,Prog Nucleic Acid Res Mol Biol.,1993,45,67}}]]. | |||
|Note=Stability:unstable in water around neutrality with a half life of about 5 min at 37°C and decomposes to PGE2, PGD2, PGF2 and 12L-hydroxy-5,8,10-heptadecatrienoic acid[[Reference:Nugteren_DH:Hazelhof_E:,Biochim. Biophys. Acta,1973,326,448|{{RelationTable/GetFirstAuthor|Reference:Nugteren_DH:Hazelhof_E:,Biochim. Biophys. Acta,1973,326,448}}]]. | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 17:31, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1601 |
| LipidMaps | LMFA03010009 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG27 |
| Prostaglandin G2 | |
|---|---|
| |
| Structural Information | |
| 7- [2R- (3S -Hydroxy-trans-1-octenyl) -(1R,4S) -5,6-dioxabicyclo [ 2.2.1 ] -heptan-3R-yl] -cis-5-heptenoic acid | |
| |
| PGG2 | |
| Formula | C20H32O6 |
| Exact Mass | 368.219888756 |
| Average Mass | 368.46448 |
| SMILES | C(CC[C@@H](OO)C=C[C@@H]([C@H]21)[C@@H](CC=CCCCC(O)=O)[C@@H](OO2)C1)CC |
| Physicochemical Information | |
| Prostaglandin G2 is produced by bis-dioxygenation and cyclization of arachidonic acid as an intermediate for the biosyntheses of various prostaglandins and thromboxanes. The responsible enzyme is prostaglandin endoperoxide synthase usually referred to as fatty acid cyclooxygenase, and distributed in various animal tissues Pace-Asciak_CR et al. . | |
Porter_NA et al.  | |
| Prostaglandin endoperoxide synthase is a bifunctional enzyme with an oxygenase activity (fatty acid cyclooxygenase) producing prostaglandin G2 from arachidonic acid and a peroxidase activity (prostagladnin hydroperoxidase) converting prostaglandin G2 to prostaglandin H2 . The enzyme is usually referred to briefly as cyclooxygenase. There are two isozymes of the enzyme. Cyclooxygenase-1 is a constitutive enzyme expressed in most mammalian cells, while cyclooxygenase-2 is a product of immediate early gene and is induced rapidly and transiently in certain types of cell by various bioactive compounds Smith_WL et al. Herschman_HR | |
| Prostaglandin G2 is a metabolic intermediate, but the compound as such has biological activities of bronchoconstriction and vasoconstriction Moncada_S et al. Chijimatsu_Y et al.. | |
| cDNA and genomic DNA each for cyclooxygenas-1 and 2 were cloned Funk_CD . | |
| Stability:unstable in water around neutrality with a half life of about 5 min at 37°C and decomposes to PGE2, PGD2, PGF2 and 12L-hydroxy-5,8,10-heptadecatrienoic acid Nugteren_DH et al.. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 13C-NMR : δ 84.6(C15) Porter_NA et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|