LBF20207PG28: Difference between revisions
No edit summary |
No edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=XPR1711 | |LipidBank=XPR1711 | ||
|LipidMaps=LMFA03010148 | |LipidMaps=LMFA03010148 | ||
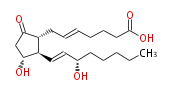
|SysName=9- | |SysName=9-Oxo- (11alpha,15S) -dihydroxy-prosta- (trans-5,trans-13) -dien-1-oic acid | ||
|Common Name=&&5-trans Prostaglandin E_2&&9- | |Common Name=&&5-trans Prostaglandin E_2&&9-Oxo- (11alpha,15S) -dihydroxy-prosta- (5E,13E) -dien-1-oic acid&& | ||
|Source=5-trans PGE2 is naturally produced by some gorgonian corals and is also a common impurity in commercial lots of PGE1. | |Source=5-trans PGE2 is naturally produced by some gorgonian corals and is also a common impurity in commercial lots of PGE1. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
Latest revision as of 17:31, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1711 |
| LipidMaps | LMFA03010148 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG28 |
| 5-trans Prostaglandin E2 | |
|---|---|
| |
| Structural Information | |
| 9-Oxo- (11α,15S) -dihydroxy-prosta- (trans-5,trans-13) -dien-1-oic acid | |
| |
| Formula | C20H32O5 |
| Exact Mass | 352.224974134 |
| Average Mass | 352.46508 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]1CC=CCCCC(O)=O)[C@@H](CC1=O)O)CC |
| Physicochemical Information | |
| 5-trans PGE2 is naturally produced by some gorgonian corals and is also a common impurity in commercial lots of PGE1. | |
| 5-trans PGE2 has 18 times potency in activating adenylate cyclase compared to PGE2. Hensby_CN et al. 5-trans PGE2 accelerates fibrinolysis by enhancing plasminogen activation mediated dby tissue-type plasminogen activator. Shimokawa_M et al. And it also inhibits platelet | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|