LBF20207PG76: Difference between revisions
New page: {{Lipid/Header}} {{Hierarchy|{{PAGENAME}}}} {{Metabolite |LipidBank=XPR8051 |LipidMaps=LMFA03010097 |SysName=Methyl- (8R,9S,11R,12R,15S) -11-Acetoxy-9,15-dihydroxyprost- (5-cis,13-trans)... |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=XPR8051 | |LipidBank=XPR8051 | ||
|LipidMaps=LMFA03010097 | |LipidMaps=LMFA03010097 | ||
|SysName=Methyl- (8R,9S,11R,12R,15S) -11-Acetoxy-9,15-dihydroxyprost- (5- | |SysName=Methyl- (8R,9S,11R,12R,15S) -11-Acetoxy-9,15-dihydroxyprost- (cis-5,trans-13) -dienoic acid | ||
|Common Name=&&Prostaglandin F_2 alpha-11-acetate methyl ester&&Methyl- (8R,9S,11R,12R,15S) -11-Acetoxy-9,15-dihydroxyprost- (5Z,13E) -dienoic acid&& | |||
|Melting Point=55°(hexane)[[Reference:Carmely_S:Kashman_Y:Loya_Y:Benayahu_Y:,Tetrahedron Lett.,1980,21,875|{{RelationTable/GetFirstAuthor|Reference:Carmely_S:Kashman_Y:Loya_Y:Benayahu_Y:,Tetrahedron Lett.,1980,21,875}}]] | |Melting Point=55°(hexane)[[Reference:Carmely_S:Kashman_Y:Loya_Y:Benayahu_Y:,Tetrahedron Lett.,1980,21,875|{{RelationTable/GetFirstAuthor|Reference:Carmely_S:Kashman_Y:Loya_Y:Benayahu_Y:,Tetrahedron Lett.,1980,21,875}}]] | ||
|Mass Spectra=CIMS m/z 411 ([M^+ +1], 1), 392(7), 350(6), 332(98),314(100),288(2),282(15). [[Reference:Carmely_S:Kashman_Y:Loya_Y:Benayahu_Y:,Tetrahedron Lett.,1980,21,875|{{RelationTable/GetFirstAuthor|Reference:Carmely_S:Kashman_Y:Loya_Y:Benayahu_Y:,Tetrahedron Lett.,1980,21,875}}]] | |Mass Spectra=CIMS m/z 411 ([M^+ +1], 1), 392(7), 350(6), 332(98),314(100),288(2),282(15). [[Reference:Carmely_S:Kashman_Y:Loya_Y:Benayahu_Y:,Tetrahedron Lett.,1980,21,875|{{RelationTable/GetFirstAuthor|Reference:Carmely_S:Kashman_Y:Loya_Y:Benayahu_Y:,Tetrahedron Lett.,1980,21,875}}]] | ||
Latest revision as of 17:49, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR8051 |
| LipidMaps | LMFA03010097 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG76 |
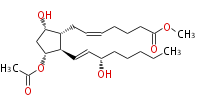
| Prostaglandin F2α-11-acetate methyl ester | |
|---|---|
| |
| Structural Information | |
| Methyl- (8R,9S,11R,12R,15S) -11-Acetoxy-9,15-dihydroxyprost- (cis-5,trans-13) -dienoic acid | |
| |
| Formula | C23H38O6 |
| Exact Mass | 410.266838948 |
| Average Mass | 410.54422 |
| SMILES | [C@@H]([C@@H]1C=C[C@H](CCCCC)O)(C[C@@H]([C@@H]1CC=CCCCC(=O)OC)O)OC(C)=O |
| Physicochemical Information | |
| 55°(hexane) Carmely_S et al. | |
| Prostaglandin F_2 alpha -11-acetate methyl ester was isolated from soft coral, Lobophyton depressum. Carmely_S et al. | |
| Spectral Information | |
| Mass Spectra | CIMS m/z 411 ([M++1], 1), 392(7), 350(6), 332(98),314(100),288(2),282(15). CarmelySet al. |
| UV Spectra | |
| IR Spectra | ν KBr max 3700, 3610, 3510, 1740, 1730, and 970 cm-1 CarmelySet al. |
| NMR Spectra | 1H-NMR(270MHz,CDCl3) δ ppm0.88(3H,t,J=6.0Hz),1.26(6H,brs),2.04(3H,s),2.32(2H,t,J=7.2Hz),2.39(1H,ddd,J=15.2,9.0,5.4Hz),2.55(1H,ddd,J=11.8,8.4,7.0Hz),3.67(3H,s),4.08(1H,q,J=6.1Hz),4.17(1H,dd,J=5.4,3.5Hz),4.90(1H,ddd,J=9.0,7.0,3.8Hz),5.41(2H,m),5.53(1H,m),5.55(1H,m). CarmelySet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|