LBF16401SC02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Common Name=&&6, 9, 12, 15-Hexadesatetraenoic acid&& | |Common Name=&&6, 9, 12, 15-Hexadesatetraenoic acid&& | ||
|Refractive=1.4870 at 29°C | |Refractive=1.4870 at 29°C | ||
|Solubility=soluble in acetone, alcohol, ether, carbon | |Solubility=soluble in acetone, alcohol, ether, carbon disulfide and petroleum ether.<!--0280--><!--0281--><!--0508--><!--0510--> | ||
|Mass Spectra=""HR-EI-MS(METHYL ESTER): M/Z; 262.19326(M) EI-MS(PYRROLIDIDE): M/Z; 154,166,194,206,260(M-41), 272(M=29), 286(M-15)EI-MS(PICOLINYL ESTER): M/Z; 339(M), 338(M-1), 324(M-15), 298(M-41), 272, 258, 232, 218, 192 ""[[Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1971,248,306|{{RelationTable/GetFirstAuthor|Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1971,248,306}}]] | |Mass Spectra=""HR-EI-MS(METHYL ESTER): M/Z; 262.19326(M) EI-MS(PYRROLIDIDE): M/Z; 154,166,194,206,260(M-41), 272(M=29), 286(M-15)EI-MS(PICOLINYL ESTER): M/Z; 339(M), 338(M-1), 324(M-15), 298(M-41), 272, 258, 232, 218, 192 ""[[Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1971,248,306|{{RelationTable/GetFirstAuthor|Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1971,248,306}}]] | ||
|NMR Spectra=""CMR(METHYL ESTER): C12, 127.093; C13, 128.939; C14, 31.529; C15, 136.736 C16: 114.741ppm PMR(METHYL ESTER): CH2=CH-C(TERMINAL OLEFINIC PROTON), 4.92-4.96, 5.62-5.88ppm(MULTIPLETS).""[[Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228|{{RelationTable/GetFirstAuthor|Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228}}]] | |NMR Spectra=""CMR(METHYL ESTER): C12, 127.093; C13, 128.939; C14, 31.529; C15, 136.736 C16: 114.741ppm PMR(METHYL ESTER): CH2=CH-C(TERMINAL OLEFINIC PROTON), 4.92-4.96, 5.62-5.88ppm(MULTIPLETS).""[[Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228|{{RelationTable/GetFirstAuthor|Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228}}]] | ||
Revision as of 15:38, 23 April 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA0205 |
| LipidMaps | LMFA01030166 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF16401SC02 |
| 6, 9, 12, 15-Hexadesatetraenoic acid | |
|---|---|
| |
| Structural Information | |
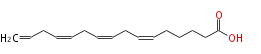
| 6, 9, 12, 15-Hexadesatetraenoic acid | |
| |
| C16:4 | |
| Formula | C16H24O2 |
| Exact Mass | 248.17763001199998 |
| Average Mass | 248.36056 |
| SMILES | C=CCC=CCC=CCC=CCCCCC(O)=O |
| Physicochemical Information | |
| 1.4870 at 29°C | |
| soluble in acetone, alcohol, ether, carbon disulfide and petroleum ether. | |
| Sardine sagax (South African pilchard); herring oil (0.7%); menhaden body oil (1.9%). Lipids from Mytilus galloprovincialis or Nitzschia pungens. It is enriched from Japanese sardine oil during purification of EPA and DHA. | |
| Spectral Information | |
| Mass Spectra | ""HR-EI-MS(METHYL ESTER): M/Z; 262.19326(M) EI-MS(PYRROLIDIDE): M/Z; 154,166,194,206,260(M-41), 272(M=29), 286(M-15)EI-MS(PICOLINYL ESTER): M/Z; 339(M), 338(M-1), 324(M-15), 298(M-41), 272, 258, 232, 218, 192 "" HayashiAet al. |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | ""CMR(METHYL ESTER): C12, 127.093; C13, 128.939; C14, 31.529; C15, 136.736 C16: 114.741ppm PMR(METHYL ESTER): CH2=CH-C(TERMINAL OLEFINIC PROTON), 4.92-4.96, 5.62-5.88ppm(MULTIPLETS)."" HayashiAet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|