LBF18303HP03: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
{{Lipid/Header}} | |||
{{Metabolite | {{Metabolite | ||
| Line 11: | Line 13: | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(cis,trans,cis-isomer)[[Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191}}]]: C10: 5.95ppm; C11: 6.54ppm; C12: 5.54ppm; C13: 4.38ppm; J10-11=11Hz; J11-12=15Hz[C11-12: trans]; J12-13=8Hz <SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(cis,trans,cis-isomer; after reduction)[[Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191}}]]: C10: 5.94ppm; C11: 6.49ppm; C12: 5.64ppm; C13: 4.20ppm | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(cis,trans,cis-isomer)[[Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191}}]]: C10: 5.95ppm; C11: 6.54ppm; C12: 5.54ppm; C13: 4.38ppm; J10-11=11Hz; J11-12=15Hz[C11-12: trans]; J12-13=8Hz <SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(cis,trans,cis-isomer; after reduction)[[Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Weisleder_D:,Lipids,1972,7,191}}]]: C10: 5.94ppm; C11: 6.49ppm; C12: 5.64ppm; C13: 4.20ppm | ||
}} | }} | ||
{{Lipid/Footer}} | |||
Revision as of 22:00, 26 July 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8052 |
| LipidMaps | LMFA01040015 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18303HP03 |
| 13-Hydroperoxy-9,11,15-Octadecatrienoic Acid | |
|---|---|
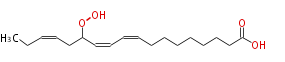
| |
| Structural Information | |
| 13-Hydroperoxy-9,11,15-Octadecatrienoic Acid/13-Hydroperoxy-9,11,15-Octadecatrienoate | |
| |
| Formula | C18H30O4 |
| Exact Mass | 310.21440944799997 |
| Average Mass | 310.4284 |
| SMILES | CCC=CCC(OO)C=CC=CCCCCCCCC(O)=O |
| Physicochemical Information | |
| Spectral Information | |
| Mass Spectra | EI-MS(Me-ester; after reduction and hydrogenation) Chan_HWS Chan_HWS et al.: m/e=243[O=CH(CH2)11C(=OH)OCH3]; 214[CH2(CH2)10C(=OH)OCH3]; 211[O=CH(CH2)11C=O], GC-EI-MS(Me-ester; after reduction and TMS) TeraoJet al. Frankel_EN et al.: m/e=380[M]; 365[M-CH3]; 311[SMTO=CH-CH=CH-CH=CH-(CH2)7-COOCH3] |
| UV Spectra | (Me-ester; after reduction; in etoh) Chan_HWS et al., cis, trans, cis isomer: lmax=233nm, trans, trans, cis isomer: lmax=232nm |
| IR Spectra | (Me-ester; after reduction) Chan_HWS et al. Frankel_EN et al., cis, trans, cis isomer: 989-983 and 950-945cm-1; trans, trans, cis isomer: 992-983cm-1, (Me-ester) ToyodaIet al., OOH group: 3400cm-1 |
| NMR Spectra | 1H-NMR(cis,trans,cis-isomer) Gardner_HW et al.: C10: 5.95ppm; C11: 6.54ppm; C12: 5.54ppm; C13: 4.38ppm; J10-11=11Hz; J11-12=15Hz[C11-12: trans]; J12-13=8Hz 1H-NMR(cis,trans,cis-isomer; after reduction) Gardner_HW et al.: C10: 5.94ppm; C11: 6.49ppm; C12: 5.64ppm; C13: 4.20ppm |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|