LBF20000SC01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 10: | Line 10: | ||
|Density=d<SUP><FONT SIZE=-1>1</FONT></SUP><SUP><FONT SIZE=-1>0</FONT></SUP><SUP><FONT SIZE=-1>0</FONT></SUP><SUB><FONT SIZE=-1>4</FONT></SUB> 0.8240 | |Density=d<SUP><FONT SIZE=-1>1</FONT></SUP><SUP><FONT SIZE=-1>0</FONT></SUP><SUP><FONT SIZE=-1>0</FONT></SUP><SUB><FONT SIZE=-1>4</FONT></SUB> 0.8240 | ||
|Optical=1.4250 at 100°C | |Optical=1.4250 at 100°C | ||
|Solubility=practically insoluble in water; sparingly soluble in cold water; freely in hot absolute alcohol, benzene, chloroform, ether and petroleum ether. | |Solubility=practically insoluble in water; sparingly soluble in cold water; freely in hot absolute alcohol, benzene, chloroform, ether and petroleum ether.[[Reference:Weitkamp_AW:Smiljanic_AM:Rothman_S:,J. Am. Chem. Soc.,1947,69,1936|{{RelationTable/GetFirstAuthor|Reference:Weitkamp_AW:Smiljanic_AM:Rothman_S:,J. Am. Chem. Soc.,1947,69,1936}}]] | ||
|Mass Spectra={{Image200|LBF20000SC01SP0001.gif}} (provided by Dr. Takeshi Kasama). | |Mass Spectra={{Image200|LBF20000SC01SP0001.gif}} (provided by Dr. Takeshi Kasama). | ||
|Chromatograms=Gas liquid chromatogram {{Image200|LBF20000SC01CH0001.gif}}{{Image200|LBF20000SC01CH0002.gif}} (provided by Dr. Akiko Horiuchi). | |Chromatograms=Gas liquid chromatogram {{Image200|LBF20000SC01CH0001.gif}}{{Image200|LBF20000SC01CH0002.gif}} (provided by Dr. Akiko Horiuchi). | ||
}} | }} | ||
Revision as of 09:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | DFA0020 |
| LipidMaps | LMFA01010020 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20000SC01 |
| Arachidic acid | |
|---|---|
| |
| Structural Information | |
| Icosanoic acid / Eicosanoic acid | |
| |
| Formula | C20H40O2 |
| Exact Mass | 312.302830524 |
| Average Mass | 312.53040000000004 |
| SMILES | C(CCCCCCCCCCCCCCCC(O)=O)CCC |
| Physicochemical Information | |
| 76.5-77.0°C | |
| 204°C at 1 mmHg | |
| d1004 0.8240 | |
| 1.4250 at 100°C | |
| practically insoluble in water; sparingly soluble in cold water; freely in hot absolute alcohol, benzene, chloroform, ether and petroleum ether. Weitkamp_AW et al. | |
| Spectral Information | |
| Mass Spectra | 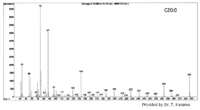 (provided by Dr. Takeshi Kasama). |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | Gas liquid chromatogram 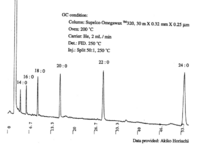 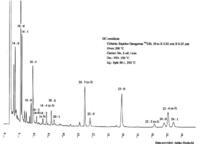 (provided by Dr. Akiko Horiuchi). |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|