LBF20207PG23: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA03010003 | |LipidMaps=LMFA03010003 | ||
|SysName=7- [ 3 (R) -Hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl-5-oxocyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid | |SysName=7- [ 3 (R) -Hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl-5-oxocyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid | ||
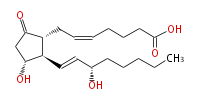
|Common Name=&&Prostaglandin E_2&& | |Common Name=&&Prostaglandin E_2&&7- [ 3 (R) -Hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl-5-oxocyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid&& | ||
|Melting Point=65-66°C [[Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397}}]] | |Melting Point=65-66°C [[Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397}}]] | ||
|Reflactive=[<FONT FACE="Symbol">a</FONT>]X<sub>D</sub><sup>26</sup>=-61.0°(C=1.0, TETRAHYDROFURAN) [[Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397}}]] | |Reflactive=[<FONT FACE="Symbol">a</FONT>]X<sub>D</sub><sup>26</sup>=-61.0°(C=1.0, TETRAHYDROFURAN) [[Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397}}]] | ||
Revision as of 09:01, 20 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR1401 |
| LipidMaps | LMFA03010003 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG23 |
| Prostaglandin E2 | |
|---|---|
| |
| Structural Information | |
| 7- [ 3 (R) -Hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl-5-oxocyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid | |
| |
| Formula | C20H32O5 |
| Exact Mass | 352.224974134 |
| Average Mass | 352.46508 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]1CC=CCCCC(O)=O)[C@@H](CC1=O)O)CC |
| Physicochemical Information | |
| 65-66°C Corey_EJ et al. | |
| ETHYL ACETATE,THF,CHLOROFORM Donaldson_RE et al. Sih_CJ et al.. STABILITIES: to be stable under neutral condition. to decompose to PGA2 under acidic and to PGB2 under basic conditions Karim_SM et al. Pike_JEet al. | |
| Spectral Information | |
| Mass Spectra | d,l-mixture ; 334(M+-18), 316, 298, 190 Chen_SML et al. |
| UV Spectra | |
| IR Spectra | d,l-mixture ; 3400, 1710, 970cm-1 Chen_SML et al. |
| NMR Spectra | 13C-NMR(CDCl3) : 214.71(C9),178.39(C1), 136.62(C14), 131.52(C13), 130.91(C5), 126.69(C6), 73.19(C15), 72.13(C11), 54.55(C12), 53.51(C8), 46.23(C10), 37.00 (C16), 33.56(C2), 31.73(C18), 26.47(C4), 25.20(C7,17), 24.60(C3), 22.64(C19), 14.04. Donaldson_RE et al.. 1H-NMR(CDCl3) : d5.67(dd, J=6.6Hz, 15.4, 1H, 14-CH), 5.57(dd, J=8.1, 15.4Hz, 1H, 13-CH), 5.40(m, 2H, 5.6-CH), 4.12(q, J=6.5, 6.7, 6.8Hz, 1H, 15-CH), 4.06(q, J=8.1, 8.2, 8.3Hz, 1H, 11-CH), 2.72(dd, J= Donaldson_RE et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|