LBF20303PG05: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|LipidBank=XPR1705 | |LipidBank=XPR1705 | ||
|LipidMaps=LMFA03010142 | |LipidMaps=LMFA03010142 | ||
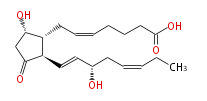
|SysName=9alpha,15S-Dihydroxy-11-oxo-prosta- | |SysName=(9alpha,15S) -Dihydroxy-11-oxo-prosta- (5-cis,13-trans,17-cis) -trien-1-oic acid | ||
|Common Name=&&Prostaglandin D_3&& | |Common Name=&&Prostaglandin D_3&&(9alpha,15S) -Dihydroxy-11-oxo-prosta- (5Z,13E,17Z) -trien-1-oic acid&& | ||
|Source=PGD3 is produced by the metabolism of EPA via the cyclooxygenase pathway.[[Reference:Kulkarni_PS:Kaufman_PL:Srinivasan_BD:,J. Ocul. Pharmacol.,1987,3,349|{{RelationTable/GetFirstAuthor|Reference:Kulkarni_PS:Kaufman_PL:Srinivasan_BD:,J. Ocul. Pharmacol.,1987,3,349}}]] | |Source=PGD3 is produced by the metabolism of EPA via the cyclooxygenase pathway.[[Reference:Kulkarni_PS:Kaufman_PL:Srinivasan_BD:,J. Ocul. Pharmacol.,1987,3,349|{{RelationTable/GetFirstAuthor|Reference:Kulkarni_PS:Kaufman_PL:Srinivasan_BD:,J. Ocul. Pharmacol.,1987,3,349}}]] | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
Revision as of 16:30, 27 May 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1705 |
| LipidMaps | LMFA03010142 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20303PG05 |
| Prostaglandin D3 | |
|---|---|
| |
| Structural Information | |
| (9α,15S) -Dihydroxy-11-oxo-prosta- (5-cis,13-trans,17-cis) -trien-1-oic acid | |
| |
| Formula | C20H30O5 |
| Exact Mass | 350.20932407 |
| Average Mass | 350.4492 |
| SMILES | C(=CC[C@@H](O)C=C[C@H]([C@H]1CC=CCCCC(O)=O)C(C[C@@H]1O)=O)CC |
| Physicochemical Information | |
| PGD3 is produced by the metabolism of EPA via the cyclooxygenase pathway. Kulkarni_PS et al. | |
| PGD3 has almost same ability to decrease systemic blood pressure in rats and to decrease intraocular pressure in rabbits. Bundy_GL et al. Goh_Y et al. Kulkarni_PS et al. However, it is 3 to 5 times more potent than PGD2 in the inhibition of ADP-induced human platelet aggregation. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|