LBF20307HO01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
|Symbol=15(S)-HETrE | |||
|Biological Activity=Compared to a series of hydroxypolyenoic fatty acids, 15(S)-HETrE is a more potent inhibitor of human 5-lipoxygenase from polymorphonuclear leukocytes having an IC<SUB><FONT SIZE=-1>5</FONT></SUB><SUB><FONT SIZE=-1>0</FONT></SUB> of 4.6<FONT FACE="Symbol">m</FONT>M at an arachidonic acid concentration of 5 <FONT FACE="Symbol">m</FONT>M [[Reference:Petrich_K:Ludwig_P:Kuhn_H:Schewe_T:,Biochem. J.,1996,314 (Pt 3),911|{{RelationTable/GetFirstAuthor|Reference:Petrich_K:Ludwig_P:Kuhn_H:Schewe_T:,Biochem. J.,1996,314 (Pt 3),911}}]]. In rat basophilic leukemia cell lysates, 15(S)-HETrE inhibits 5-lypoxygenase with an IC<SUB><FONT SIZE=-1>5</FONT></SUB><SUB><FONT SIZE=-1>0</FONT></SUB> of 77<FONT FACE="Symbol">m</FONT>M (arachidonic acid concentration of 60<FONT FACE="Symbol">m</FONT>M) [[Reference:Haviv_F:Ratajczyk_JD:DeNet_RW:Martin_YC:Dyer_RD:Carter_GW:,J. Med. Chem.,1987,30,254|{{RelationTable/GetFirstAuthor|Reference:Haviv_F:Ratajczyk_JD:DeNet_RW:Martin_YC:Dyer_RD:Carter_GW:,J. Med. Chem.,1987,30,254}}]]. 15(S)-HETrE is 10- to 20-fold less potent than 15(S)-HETE and 15(S)-HpETE under these experimental conditions [[Reference:Haviv_F:Ratajczyk_JD:DeNet_RW:Martin_YC:Dyer_RD:Carter_GW:,J. Med. Chem.,1987,30,254|{{RelationTable/GetFirstAuthor|Reference:Haviv_F:Ratajczyk_JD:DeNet_RW:Martin_YC:Dyer_RD:Carter_GW:,J. Med. Chem.,1987,30,254}}]]. | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 06:00, 7 January 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8144 |
| LipidMaps | LMFA03050007 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20307HO01 |
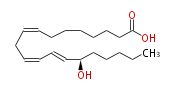
| 15S-hydroxy-8Z,11Z,13E-eicosatrienoic acid | |
|---|---|
| |
| Structural Information | |
| 15S-hydroxy-8Z,11Z,13E-eicosatrienoic acid | |
| |
| 15(S)-HETrE | |
| Formula | C20H34O3 |
| Exact Mass | 322.25079495399996 |
| Average Mass | 322.48216 |
| SMILES | C(CCC(C=CC=CCC=CCCCCCCC(O)=O)O)CC |
| Physicochemical Information | |
| 15(S)-HETrE is a hydroxy fatty acid derived from dihomo-g-linolenic acid. | |
| Compared to a series of hydroxypolyenoic fatty acids, 15(S)-HETrE is a more potent inhibitor of human 5-lipoxygenase from polymorphonuclear leukocytes having an IC50 of 4.6mM at an arachidonic acid concentration of 5 mM Petrich_K et al.. In rat basophilic leukemia cell lysates, 15(S)-HETrE inhibits 5-lypoxygenase with an IC50 of 77mM (arachidonic acid concentration of 60mM) Haviv_F et al.. 15(S)-HETrE is 10- to 20-fold less potent than 15(S)-HETE and 15(S)-HpETE under these experimental conditions Haviv_F et al.. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | lmax: 235nm e: 23,000 |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|