LBF20406HO21: Difference between revisions
No edit summary |
No edit summary |
||
| Line 15: | Line 15: | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(250MHz, ACETONE-D<SUB><FONT SIZE=-1>6</FONT></SUB>) ; <FONT FACE="Symbol">d</FONT> 6.58(dd, J=15.3, 11.0Hz, 1H, 10-CH), 5.97(t, J=11.0Hz, 1H, 9-CH), 5.72(dd, J=15.3, 6.2 Hz, 1H, 11-CH), 5.29(m, 5H, 5,6,8,14,15-CH), 4.16(q, J=6.3Hz, 1H, 12-CH), 2.94(t, J=6.1Hz, 2H, 7-CH), 2.27(t,J= 7.4Hz, 2H,2-CH), 2.22(m, 2H, 13-CH), 1.66 and 2.14(m, 2H, 4-and 16-CH), 0.87(t, J=6.3Hz, 3H, 20-CH) [[Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789|{{RelationTable/GetFirstAuthor|Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789}}]]. <SUP><FONT SIZE=-1>1</FONT></SUP><SUP><FONT SIZE=-1>3</FONT></SUP>NMR(C<SUB><FONT SIZE=-1>6</FONT></SUB>D<SUB><FONT SIZE=-1>6</FONT></SUB>) : 174.3, 137.77, 132.02, 129.98, 129.62, 128.88, 128.69, 125.96, 124.44 [[Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789|{{RelationTable/GetFirstAuthor|Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789}}]] | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(250MHz, ACETONE-D<SUB><FONT SIZE=-1>6</FONT></SUB>) ; <FONT FACE="Symbol">d</FONT> 6.58(dd, J=15.3, 11.0Hz, 1H, 10-CH), 5.97(t, J=11.0Hz, 1H, 9-CH), 5.72(dd, J=15.3, 6.2 Hz, 1H, 11-CH), 5.29(m, 5H, 5,6,8,14,15-CH), 4.16(q, J=6.3Hz, 1H, 12-CH), 2.94(t, J=6.1Hz, 2H, 7-CH), 2.27(t,J= 7.4Hz, 2H,2-CH), 2.22(m, 2H, 13-CH), 1.66 and 2.14(m, 2H, 4-and 16-CH), 0.87(t, J=6.3Hz, 3H, 20-CH) [[Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789|{{RelationTable/GetFirstAuthor|Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789}}]]. <SUP><FONT SIZE=-1>1</FONT></SUP><SUP><FONT SIZE=-1>3</FONT></SUP>NMR(C<SUB><FONT SIZE=-1>6</FONT></SUB>D<SUB><FONT SIZE=-1>6</FONT></SUB>) : 174.3, 137.77, 132.02, 129.98, 129.62, 128.88, 128.69, 125.96, 124.44 [[Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789|{{RelationTable/GetFirstAuthor|Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789}}]] | ||
|Source= | |Source= | ||
|Chemical Synthesis=[[Reference:Corey_EJ:Niwa_H:Knolle_J:,J. Am. Chem. Soc.,1978,100,1942|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Niwa_H:Knolle_J:,J. Am. Chem. Soc.,1978,100,1942}}]] | |Chemical Synthesis=[[Reference:Corey_EJ:Niwa_H:Knolle_J:,J. Am. Chem. Soc.,1978,100,1942|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Niwa_H:Knolle_J:,J. Am. Chem. Soc.,1978,100,1942}}]] {{Image200|LBF20406HO21FT0001.gif}} | ||
|Metabolism=When arachidonic acid is oxygenated by 12-lipoxygenase, 12(S)-hydroperoxy-5,8,10,14-(Z,Z,E,Z)-eicosatetraenoic acid is produced [[Reference:Yamamoto_S:Suzuki_H:Ueda_N:,Prog. Lipid Res.,1997,36,23|{{RelationTable/GetFirstAuthor|Reference:Yamamoto_S:Suzuki_H:Ueda_N:,Prog. Lipid Res.,1997,36,23}}]] | |Metabolism=When arachidonic acid is oxygenated by 12-lipoxygenase, 12(S)-hydroperoxy-5,8,10,14-(Z,Z,E,Z)-eicosatetraenoic acid is produced [[Reference:Yamamoto_S:Suzuki_H:Ueda_N:,Prog. Lipid Res.,1997,36,23|{{RelationTable/GetFirstAuthor|Reference:Yamamoto_S:Suzuki_H:Ueda_N:,Prog. Lipid Res.,1997,36,23}}]]. The latter compound is reduced to a corresponding 12(S)-hydroxy acid with whole cells or crude enzyme preparations. | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 20:55, 25 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR6102 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406HO21 |
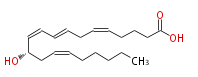
| 12 (S) -Hydoxy-5,8,10,14- (Z,E,Z,Z) -eicosatetraenoic acid | |
|---|---|
| |
| Structural Information | |
| 12 (S) -Hydoxy-5,8,10,14- (Z,E,Z,Z) -eicosatetraenoic acid | |
| |
| Formula | C20H32O3 |
| Exact Mass | 320.23514489 |
| Average Mass | 320.46628 |
| SMILES | C(CC=CC[C@@H](C=CC=CCC=CCCCC(O)=O)O)CCC |
| Physicochemical Information | |
| DIETHYL ETHER , ACETONE , BENZENE LeblancYet al. | |
Corey_EJ et al.  | |
| When arachidonic acid is oxygenated by 12-lipoxygenase, 12(S)-hydroperoxy-5,8,10,14-(Z,Z,E,Z)-eicosatetraenoic acid is produced Yamamoto_S et al.. The latter compound is reduced to a corresponding 12(S)-hydroxy acid with whole cells or crude enzyme preparations. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER ; m/e 316, 303, 223, 191, 141, 107(base peak) JustGet al. |
| UV Spectra | METHYL ESTER ; l MeOHmax = 234nm JustGet al.METHYL ESTER ; l EtOHmax = 237nm(e 30500) HambergMet al. |
| IR Spectra | NEAT : n 3480b, 1710, 1460, 1400cm-1 LeblancYet al. |
| NMR Spectra | 1H-NMR(250MHz, ACETONE-D6) ; d 6.58(dd, J=15.3, 11.0Hz, 1H, 10-CH), 5.97(t, J=11.0Hz, 1H, 9-CH), 5.72(dd, J=15.3, 6.2 Hz, 1H, 11-CH), 5.29(m, 5H, 5,6,8,14,15-CH), 4.16(q, J=6.3Hz, 1H, 12-CH), 2.94(t, J=6.1Hz, 2H, 7-CH), 2.27(t,J= 7.4Hz, 2H,2-CH), 2.22(m, 2H, 13-CH), 1.66 and 2.14(m, 2H, 4-and 16-CH), 0.87(t, J=6.3Hz, 3H, 20-CH) LeblancYet al.. 13NMR(C6D6) : 174.3, 137.77, 132.02, 129.98, 129.62, 128.88, 128.69, 125.96, 124.44 LeblancYet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|