LBF16304SC01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA01030136 | |LipidMaps=LMFA01030136 | ||
|SysName=6, 9, 12-Hexadecatrienoic acid | |SysName=6, 9, 12-Hexadecatrienoic acid | ||
|Common Name=&&6, 9, 12-Hexadecatrienoic acid&& | |||
|Solubility=soluble in alcohol and ether.[[Reference:Klenk_E:Steinbach_H:,Hoppe Seylers Z. Physiol. Chem.,1959,316,31|{{RelationTable/GetFirstAuthor|Reference:Klenk_E:Steinbach_H:,Hoppe Seylers Z. Physiol. Chem.,1959,316,31}}]][[Reference:Stoffel_W:Ahrens_EH_Jr:,J. Am. Chem. Soc.,1958,80,6604|{{RelationTable/GetFirstAuthor|Reference:Stoffel_W:Ahrens_EH_Jr:,J. Am. Chem. Soc.,1958,80,6604}}]][[Reference:Stoffel_W:Ahrens_EH_Jr:,J. Lipid Res.,1959,1,139|{{RelationTable/GetFirstAuthor|Reference:Stoffel_W:Ahrens_EH_Jr:,J. Lipid Res.,1959,1,139}}]] | |Solubility=soluble in alcohol and ether.[[Reference:Klenk_E:Steinbach_H:,Hoppe Seylers Z. Physiol. Chem.,1959,316,31|{{RelationTable/GetFirstAuthor|Reference:Klenk_E:Steinbach_H:,Hoppe Seylers Z. Physiol. Chem.,1959,316,31}}]][[Reference:Stoffel_W:Ahrens_EH_Jr:,J. Am. Chem. Soc.,1958,80,6604|{{RelationTable/GetFirstAuthor|Reference:Stoffel_W:Ahrens_EH_Jr:,J. Am. Chem. Soc.,1958,80,6604}}]][[Reference:Stoffel_W:Ahrens_EH_Jr:,J. Lipid Res.,1959,1,139|{{RelationTable/GetFirstAuthor|Reference:Stoffel_W:Ahrens_EH_Jr:,J. Lipid Res.,1959,1,139}}]] | ||
|Mass Spectra=HR-EI-MS (METHYL ESTER) : M/Z; 264.20910(M) 001) EI-MS (PYRROLIDIDE) : M/Z; 154, 166, 194, 206, 234, 246[[Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228|{{RelationTable/GetFirstAuthor|Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228}}]]. | |Mass Spectra=HR-EI-MS (METHYL ESTER) : M/Z; 264.20910(M) 001) EI-MS (PYRROLIDIDE) : M/Z; 154, 166, 194, 206, 234, 246[[Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228|{{RelationTable/GetFirstAuthor|Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228}}]]. | ||
|NMR Spectra=CMR (METHYL ESTER) : C12, 127.797; C13, 130.127; C14, 29.308; C15, 22.753; C16: 13.760ppm [[Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228|{{RelationTable/GetFirstAuthor|Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228}}]] PMR(METHYL ESTER): CH3-C-C-C=C- (TERMINAL METHYL GROUP) , 0.83-0.98ppm (TRIPLETS) [[Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228|{{RelationTable/GetFirstAuthor|Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228}}]] | |NMR Spectra=CMR (METHYL ESTER) : C12, 127.797; C13, 130.127; C14, 29.308; C15, 22.753; C16: 13.760ppm [[Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228|{{RelationTable/GetFirstAuthor|Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228}}]] PMR(METHYL ESTER): CH3-C-C-C=C- (TERMINAL METHYL GROUP) , 0.83-0.98ppm (TRIPLETS) [[Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228|{{RelationTable/GetFirstAuthor|Reference:Hayashi_A:Matsubara_T:,Biochim. Biophys. Acta,1970,202,228}}]] | ||
}} | }} | ||
Revision as of 09:01, 20 December 2008
| IDs and Links | |
|---|---|
| LipidBank | DFA0175 |
| LipidMaps | LMFA01030136 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF16304SC01 |
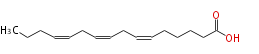
| 6, 9, 12-Hexadecatrienoic acid | |
|---|---|
| |
| Structural Information | |
| 6, 9, 12-Hexadecatrienoic acid | |
| |
| Formula | C16H26O2 |
| Exact Mass | 250.19328007599998 |
| Average Mass | 250.37643999999997 |
| SMILES | CCCC=CCC=CCC=CCCCCC(O)=O |
| Physicochemical Information | |
| soluble in alcohol and ether. KlenkEet al. StoffelWet al. StoffelWet al. | |
| Spectral Information | |
| Mass Spectra | HR-EI-MS (METHYL ESTER) : M/Z; 264.20910(M) 001) EI-MS (PYRROLIDIDE) : M/Z; 154, 166, 194, 206, 234, 246 HayashiAet al.. |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | CMR (METHYL ESTER) : C12, 127.797; C13, 130.127; C14, 29.308; C15, 22.753; C16: 13.760ppm HayashiAet al. PMR(METHYL ESTER): CH3-C-C-C=C- (TERMINAL METHYL GROUP) , 0.83-0.98ppm (TRIPLETS) HayashiAet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|