LBF18109EO01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA01070002 | |LipidMaps=LMFA01070002 | ||
|SysName=12,13-Epoxy-9-Octadecenoic Acid | |SysName=12,13-Epoxy-9-Octadecenoic Acid | ||
|Common Name=&&12,13-Epoxy-9-Octadecenoic Acid&& | |||
|Mass Spectra=GC-EI-MS(after solvolysation-trimethylsilylation in MeOH)[[Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31|{{RelationTable/GetFirstAuthor|Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31}}]][[Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971|{{RelationTable/GetFirstAuthor|Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]]: m/e= 299[SNTO=CH-CH2-CH=CH(CH2)7COOCH3], 217[CH3(CH2)4CH(OCH3)CHOTMS], 195[OHCCH2CH=CH(CH2)7CO], 173[ SMTO=CH(CH2)4CH3] | |Mass Spectra=GC-EI-MS(after solvolysation-trimethylsilylation in MeOH)[[Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31|{{RelationTable/GetFirstAuthor|Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31}}]][[Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971|{{RelationTable/GetFirstAuthor|Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]]: m/e= 299[SNTO=CH-CH2-CH=CH(CH2)7COOCH3], 217[CH3(CH2)4CH(OCH3)CHOTMS], 195[OHCCH2CH=CH(CH2)7CO], 173[ SMTO=CH(CH2)4CH3] | ||
|IR Spectra=Trans olefin(960cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), cis olefin(720cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), trans epoxide(885cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), cis epoxide(840 and 820cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>)[[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]] | |IR Spectra=Trans olefin(960cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), cis olefin(720cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), trans epoxide(885cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), cis epoxide(840 and 820cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>)[[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR[[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]]: C8(2.01ppm), C9, 10(5.45ppm), C2, 11(2.29ppm), C12, 13(2.91ppm), J9-10= 10Hz(cis olefin) | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR[[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]]: C8(2.01ppm), C9, 10(5.45ppm), C2, 11(2.29ppm), C12, 13(2.91ppm), J9-10= 10Hz(cis olefin) | ||
}} | }} | ||
Revision as of 17:58, 19 December 2008
| IDs and Links | |
|---|---|
| LipidBank | DFA8008 |
| LipidMaps | LMFA01070002 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18109EO01 |
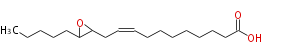
| 12,13-Epoxy-9-Octadecenoic Acid | |
|---|---|
| |
| Structural Information | |
| 12,13-Epoxy-9-Octadecenoic Acid | |
| |
| Formula | C18H32O3 |
| Exact Mass | 296.23514489 |
| Average Mass | 296.44488 |
| SMILES | C(C(CC=CCCCCCCCC(O)=O)1)(CCCCC)O1 |
| Physicochemical Information | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after solvolysation-trimethylsilylation in MeOH) KleimanRet al. Wu_GS et al. Sessa_DJ et al.: m/e= 299[SNTO=CH-CH2-CH=CH(CH2)7COOCH3], 217[CH3(CH2)4CH(OCH3)CHOTMS], 195[OHCCH2CH=CH(CH2)7CO], 173[ SMTO=CH(CH2)4CH3] |
| UV Spectra | |
| IR Spectra | Trans olefin(960cm-1), cis olefin(720cm-1), trans epoxide(885cm-1), cis epoxide(840 and 820cm-1) Sessa_DJ et al. |
| NMR Spectra | 1H-NMR Sessa_DJ et al.: C8(2.01ppm), C9, 10(5.45ppm), C2, 11(2.29ppm), C12, 13(2.91ppm), J9-10= 10Hz(cis olefin) |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|