LBF18203EO02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA01070013 | |LipidMaps=LMFA01070013 | ||
|SysName=Methyl-12,13-Epoxy-9,15-Octadecadienoate | |SysName=Methyl-12,13-Epoxy-9,15-Octadecadienoate | ||
|Mass Spectra=GC-EI-MS | |Mass Spectra=GC-EI-MS[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780}}]]: m/e=308[M]; 277[M-OCH3]; 211[CH-(O)-CHCH2CH=CH(CH2)7COOCH3-28] 83[M-CH2CH=CH(CH2)7COOCH3-28], GC-EI-MS(after hydrogenation)(105): m/e=281[M-OCH3]; 241[CH-(O)-CH(CH2)10CO OCH3]; 213[214-28]; 113[M-(CH2)10COOCH3]; 85[113-28] | ||
|IR Spectra=Cis unsaturation: 3002cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | |IR Spectra=Cis unsaturation: 3002cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439}}]]: cis unsaturationS: 5.43ppm[4H]; cis epoxide ring:2.79 AND 2.98ppm[2H] | ||
}} | }} | ||
Revision as of 09:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | DFA8072 |
| LipidMaps | LMFA01070013 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18203EO02 |
| GlcNAca/b1-3Xyla-4Galb1-3GalNAca1-4(NeuAc?1-2NeuGc4Mea1-3)GalNAcb1-4(EtnP-6)GlcNAcb1-3Manb1-4Glcb1-1Cer | |
|---|---|
| |
| Structural Information | |
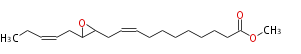
| Methyl-12,13-Epoxy-9,15-Octadecadienoate | |
| Formula | C19H32O3 |
| Exact Mass | 308.23514489 |
| Average Mass | 308.45558 |
| SMILES | C(C(CC=CCCCCCCCC(=O)OC)1)(CC=CCC)O1 |
| Physicochemical Information | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS Neff_WE et al.: m/e=308[M]; 277[M-OCH3]; 211[CH-(O)-CHCH2CH=CH(CH2)7COOCH3-28] 83[M-CH2CH=CH(CH2)7COOCH3-28], GC-EI-MS(after hydrogenation)(105): m/e=281[M-OCH3]; 241[CH-(O)-CH(CH2)10CO OCH3]; 213[214-28]; 113[M-(CH2)10COOCH3]; 85[113-28] |
| UV Spectra | |
| IR Spectra | Cis unsaturation: 3002cm-1 Neff_WE et al. |
| NMR Spectra | 1H-NMR Neff_WE et al.: cis unsaturationS: 5.43ppm[4H]; cis epoxide ring:2.79 AND 2.98ppm[2H] |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|