LBF18304HP01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA01040016 | |LipidMaps=LMFA01040016 | ||
|SysName=16-Hydroperoxy-9,12,14-Octadecatrienoic Acid/16-Hydroperoxy-9,12,14-Octadecatrienoate | |SysName=16-Hydroperoxy-9,12,14-Octadecatrienoic Acid/16-Hydroperoxy-9,12,14-Octadecatrienoate | ||
|Common Name=&&16-Hydroperoxy-9,12,14-Octadecatrienoic Acid | |Common Name=&&16-Hydroperoxy-9,12,14-Octadecatrienoic Acid&&16-Hydroperoxy-9,12,14-Octadecatrienoate&& | ||
|Mass Spectra=EI-MS(Me-ester; after reduction and hydrogenation)[[Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055}}]]: m/e=285[O=CH(CH2)14C(=OH)OCH3]; 256[CH2(CH2)13C(=OH)OCH3]; 253[O=CH(CH2)14C=O], GC-EI-MS(Me-ester; after reduction,tms)[[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055}}]]: m/e=380[M]; 365[M-CH3]; 351[SMTO=CH-CH=CH-CH=CH-CH2-CH=CH-(CH2)7-COOCH3] | |Mass Spectra=EI-MS(Me-ester; after reduction and hydrogenation)[[Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055}}]]: m/e=285[O=CH(CH2)14C(=OH)OCH3]; 256[CH2(CH2)13C(=OH)OCH3]; 253[O=CH(CH2)14C=O], GC-EI-MS(Me-ester; after reduction,tms)[[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055}}]]: m/e=380[M]; 365[M-CH3]; 351[SMTO=CH-CH=CH-CH=CH-CH2-CH=CH-(CH2)7-COOCH3] | ||
|UV Spectra=(Me-ester; after reduction; in etoh)[[Reference:Chan_HWS:Levett_G:,Lipids,1977,12,837|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:Levett_G:,Lipids,1977,12,837}}]], cis, cis, trans isomer: <FONT FACE="Symbol">l</FONT>max=236nm, cis, trans, trans isomer: <FONT FACE="Symbol">l</FONT>max=232nm | |UV Spectra=(Me-ester; after reduction; in etoh)[[Reference:Chan_HWS:Levett_G:,Lipids,1977,12,837|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:Levett_G:,Lipids,1977,12,837}}]], cis, cis, trans isomer: <FONT FACE="Symbol">l</FONT>max=236nm, cis, trans, trans isomer: <FONT FACE="Symbol">l</FONT>max=232nm | ||
|IR Spectra=(Me-ester; after reduction)[[Reference:Chan_HWS:Levett_G:,Lipids,1977,12,837|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:Levett_G:,Lipids,1977,12,837}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055}}]], cis, cis, trans isomer: 989-983 and 951-945cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>; cis, trans, trans isomer: 991-983cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>, (Me-ester)[[Reference:Toyoda_I:Terao_J:Matsushita_S:,Lipids,1982,17,84|{{RelationTable/GetFirstAuthor|Reference:Toyoda_I:Terao_J:Matsushita_S:,Lipids,1982,17,84}}]], OOH group: 3400cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | |IR Spectra=(Me-ester; after reduction)[[Reference:Chan_HWS:Levett_G:,Lipids,1977,12,837|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:Levett_G:,Lipids,1977,12,837}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055}}]], cis, cis, trans isomer: 989-983 and 951-945cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>; cis, trans, trans isomer: 991-983cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>, (Me-ester)[[Reference:Toyoda_I:Terao_J:Matsushita_S:,Lipids,1982,17,84|{{RelationTable/GetFirstAuthor|Reference:Toyoda_I:Terao_J:Matsushita_S:,Lipids,1982,17,84}}]], OOH group: 3400cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | ||
}} | }} | ||
Revision as of 09:02, 20 December 2008
| IDs and Links | |
|---|---|
| LipidBank | DFA8053 |
| LipidMaps | LMFA01040016 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18304HP01 |
| 16-Hydroperoxy-9,12,14-Octadecatrienoic Acid | |
|---|---|
| |
| Structural Information | |
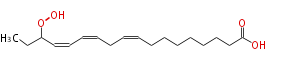
| 16-Hydroperoxy-9,12,14-Octadecatrienoic Acid/16-Hydroperoxy-9,12,14-Octadecatrienoate | |
| |
| Formula | C18H30O4 |
| Exact Mass | 310.21440944799997 |
| Average Mass | 310.4284 |
| SMILES | CCC(OO)C=CC=CCC=CCCCCCCCC(O)=O |
| Physicochemical Information | |
| Spectral Information | |
| Mass Spectra | EI-MS(Me-ester; after reduction and hydrogenation) Chan_HWS Frankel_EN et al.: m/e=285[O=CH(CH2)14C(=OH)OCH3]; 256[CH2(CH2)13C(=OH)OCH3]; 253[O=CH(CH2)14C=O], GC-EI-MS(Me-ester; after reduction,tms) Frankel_EN et al.: m/e=380[M]; 365[M-CH3]; 351[SMTO=CH-CH=CH-CH=CH-CH2-CH=CH-(CH2)7-COOCH3] |
| UV Spectra | (Me-ester; after reduction; in etoh) Chan_HWS et al., cis, cis, trans isomer: lmax=236nm, cis, trans, trans isomer: lmax=232nm |
| IR Spectra | (Me-ester; after reduction) Chan_HWS et al. Frankel_EN et al., cis, cis, trans isomer: 989-983 and 951-945cm-1; cis, trans, trans isomer: 991-983cm-1, (Me-ester) ToyodaIet al., OOH group: 3400cm-1 |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|