LBF20207TX02: Difference between revisions
m LBF20307TX02 moved to LBF20207TX02 |
m LBF20307TX02 moved to LBF20207TX02 |
||
| Line 9: | Line 9: | ||
|Common Name=&&THROMBOXANE B2&&7- [ Tetrahydro-4 (S) ,6-dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) -2H-pyran-3 (S) -yl ] -5 (Z) -heptenoic acid&& | |Common Name=&&THROMBOXANE B2&&7- [ Tetrahydro-4 (S) ,6-dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) -2H-pyran-3 (S) -yl ] -5 (Z) -heptenoic acid&& | ||
|Melting Point=95-96°C [[Reference:Hanessian_S:Lavallee_P:,Can. J. Chem.,1977,55,562|{{RelationTable/GetFirstAuthor|Reference:Hanessian_S:Lavallee_P:,Can. J. Chem.,1977,55,562}}]] | |Melting Point=95-96°C [[Reference:Hanessian_S:Lavallee_P:,Can. J. Chem.,1977,55,562|{{RelationTable/GetFirstAuthor|Reference:Hanessian_S:Lavallee_P:,Can. J. Chem.,1977,55,562}}]] | ||
|Optical=[ alpha ]X^ | |Optical=[ alpha ]X^{25}_D =57.4°(C=0.26 ETHYL ACETATE) [[Reference:Hanessian_S:Lavallee_P:,Can. J. Chem.,1977,55,562|{{RelationTable/GetFirstAuthor|Reference:Hanessian_S:Lavallee_P:,Can. J. Chem.,1977,55,562}}]] | ||
|Solubility=ETHYL ACETATE | |Solubility=ETHYL ACETATE | ||
|Mass Spectra=m/e 335, 317 [[Reference:Hanessian_S:Lavallee_P:,Can. J. Chem.,1977,55,562|{{RelationTable/GetFirstAuthor|Reference:Hanessian_S:Lavallee_P:,Can. J. Chem.,1977,55,562}}]] | |Mass Spectra=m/e 335, 317 [[Reference:Hanessian_S:Lavallee_P:,Can. J. Chem.,1977,55,562|{{RelationTable/GetFirstAuthor|Reference:Hanessian_S:Lavallee_P:,Can. J. Chem.,1977,55,562}}]] | ||
|IR Spectra=FILM: nu 3380, 1705cm^- | |IR Spectra=FILM: nu 3380, 1705cm^{-1} [[Reference:Hanessian_S:Lavallee_P:,Can. J. Chem.,1977,55,562|{{RelationTable/GetFirstAuthor|Reference:Hanessian_S:Lavallee_P:,Can. J. Chem.,1977,55,562}}]] | ||
|NMR Spectra=^1 H-NMR(CDCl_3 ) : delta 5.86(m, 1H, 14-CH), 5.72(m, 1H, 13-CH), 5.46(m, 2H, 5,6-CH), 5.35 and 5.23(m, 1H, 11-CH), 4.41(m, 1H, 12-CH), 4.22(m, 1H, 15-CH), 4.11(m, 1H, 9-CH), 2.35(t, 2H, 2-CH_2 ), 0.89(m, 3H, 20-CH_3 ) [[Reference:Kotovych_G:Aarts_GHM:,Can. J. Chem.,1980,58,1111|{{RelationTable/GetFirstAuthor|Reference:Kotovych_G:Aarts_GHM:,Can. J. Chem.,1980,58,1111}}]] | |NMR Spectra=^1 H-NMR(CDCl_3 ) : delta 5.86(m, 1H, 14-CH), 5.72(m, 1H, 13-CH), 5.46(m, 2H, 5,6-CH), 5.35 and 5.23(m, 1H, 11-CH), 4.41(m, 1H, 12-CH), 4.22(m, 1H, 15-CH), 4.11(m, 1H, 9-CH), 2.35(t, 2H, 2-CH_2 ), 0.89(m, 3H, 20-CH_3 ) [[Reference:Kotovych_G:Aarts_GHM:,Can. J. Chem.,1980,58,1111|{{RelationTable/GetFirstAuthor|Reference:Kotovych_G:Aarts_GHM:,Can. J. Chem.,1980,58,1111}}]] | ||
|Source=Thromboxane B2 as a stable degradation product of bioactive but unstable thromboxane A2 is detected in the tissue where thromboxane A2 is produced [[Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293|{{RelationTable/GetFirstAuthor|Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293}}]]. | |Source=Thromboxane B2 as a stable degradation product of bioactive but unstable thromboxane A2 is detected in the tissue where thromboxane A2 is produced [[Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293|{{RelationTable/GetFirstAuthor|Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293}}]]. | ||
Revision as of 19:00, 25 February 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR2101 |
| LipidMaps | LMFA03030002 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207TX02 |
| THROMBOXANE B2 | |
|---|---|
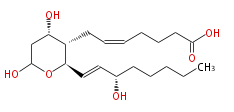
| |
| Structural Information | |
| 7- [ Tetrahydro-4 (S) ,6-dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) -2H-pyran-3 (S) -yl ] -5 (Z) -heptenoic acid | |
| |
| TXB2 | |
| Formula | C20H34O6 |
| Exact Mass | 370.23553882 |
| Average Mass | 370.48036 |
| SMILES | C(CC[C@H](O)C=C[C@@H](O1)[C@@H]([C@@H](CC1O)O)CC=CCCCC(O)=O)CC |
| Physicochemical Information | |
| 95-96°C Hanessian_S et al. | |
| [ α ]X25 D =57.4°(C=0.26 ETHYL ACETATE) HanessianSet al. | |
| ETHYL ACETATE | |
| Thromboxane B2 as a stable degradation product of bioactive but unstable thromboxane A2 is detected in the tissue where thromboxane A2 is produced Moncada_S et al.. | |
Hanessian_S et al.  | |
| The major urinary metabolite of tromboxane B2 is 2,3-dinor-thromboxane B2 Kindahl_H , and 11-dehydro-thromboxane B2 is known as a suitble parameter for monitoring thromboxane production in human Westlund_P et al.. 11-Hydroxythromboxane B2 dehydrogenase responsible for the 11-dehydro-thromboxane B2 production was identified as cytosolic aldehyde dehydrogenase Westlund_P et al.. | |
| Thromboxane B2 as s stable degradation product of thromboxane A2 shows diminishd biological activity Whittle_BJ et al.. | |
| Spectral Information | |
| Mass Spectra | m/e 335, 317 HanessianSet al. |
| UV Spectra | |
| IR Spectra | FILM: ν 3380, 1705cm-1 HanessianSet al. |
| NMR Spectra | 1H-NMR(CDCl3) : δ 5.86(m, 1H, 14-CH), 5.72(m, 1H, 13-CH), 5.46(m, 2H, 5,6-CH), 5.35 and 5.23(m, 1H, 11-CH), 4.41(m, 1H, 12-CH), 4.22(m, 1H, 15-CH), 4.11(m, 1H, 9-CH), 2.35(t, 2H, 2-CH2), 0.89(m, 3H, 20-CH3) KotovychGet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|