LBF20406CV02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Common Name=&&clavulolactone III&&(R) -4-{ (1E,3Z) -3- [ (S) -2-acetoxy-2- [ (Z) -2-octenyl ] -5-oxo-3-cyclopentenylidene ] -1-propenyl}-4-butanolide&& | |Common Name=&&clavulolactone III&&(R) -4-{ (1E,3Z) -3- [ (S) -2-acetoxy-2- [ (Z) -2-octenyl ] -5-oxo-3-cyclopentenylidene ] -1-propenyl}-4-butanolide&& | ||
|Optical=[ alpha ]_D -7.3°(C 0.21, CHCl_3 )[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | |Optical=[ alpha ]_D -7.3°(C 0.21, CHCl_3 )[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | ||
|Mass Spectra=EIMS m/z 372 (M^+ ). HREIMS m/z 372.1961 for | |Mass Spectra=EIMS m/z 372 (M^+ ). HREIMS m/z 372.1961 for C_{22}H_{28}O_5 , calcd 372.1937.[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | ||
|UV Spectra= lambda ^ | |UV Spectra= lambda ^{EtOH}_{max} 293 nm( epsilon 15500),229 nm( epsilon 17900)[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | ||
|IR Spectra= nu ^ | |IR Spectra= nu ^{film}_{max}1778,1742, 1698, 1643, and 1232cm^{-1}[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | ||
|NMR Spectra=^1 H-NMR(400MHz,CDCl_3 ) delta ppm0.88(3H,t,J=7.1Hz),1.22-1.33(6H,m),1.97(2H,brq,J=7.2Hz),2.03(3H,s),2.05(1H,m),2.47(1H,m),2.58(2H,dd,J=6.9,9.3Hz),2.65(1H,brdd,J=7.6,14.3Hz),2.84(1H,brdd,J=7.3,14.3Hz),5.10(1H,brdd,J=7.3,7.9Hz),5.22(1H,m),5.54(1H,m),6.08(1H,dd,J=7.3,15.6Hz),6.38(1H,d,J=6.1Hz),6.53(1H,d,J=11.3Hz),7.49(1H,d,J=6.1Hz),7.82(1H,brdd,J=11.3,15.6Hz).[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] ^ | |NMR Spectra=^1 H-NMR(400MHz,CDCl_3 ) delta ppm0.88(3H,t,J=7.1Hz),1.22-1.33(6H,m),1.97(2H,brq,J=7.2Hz),2.03(3H,s),2.05(1H,m),2.47(1H,m),2.58(2H,dd,J=6.9,9.3Hz),2.65(1H,brdd,J=7.6,14.3Hz),2.84(1H,brdd,J=7.3,14.3Hz),5.10(1H,brdd,J=7.3,7.9Hz),5.22(1H,m),5.54(1H,m),6.08(1H,dd,J=7.3,15.6Hz),6.38(1H,d,J=6.1Hz),6.53(1H,d,J=11.3Hz),7.49(1H,d,J=6.1Hz),7.82(1H,brdd,J=11.3,15.6Hz).[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] ^{13}C-NMR(100MHz,CDCl_3 ) delta ppm14.0,21.6,22.5,27.4,28.6,28.6,29.0,31.4,35.7,80.0,85.0,121.1,127.6,132.4,135.0,136.6,139.8,139.8,156.3,169.6,176.5,194.0.[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | ||
|Source=Clavulolactones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard.[[Reference:Iwashima_M:Okamoto_K:Konno_F:Iguchi_K:,J. Nat. Prod.,1999,62,352|{{RelationTable/GetFirstAuthor|Reference:Iwashima_M:Okamoto_K:Konno_F:Iguchi_K:,J. Nat. Prod.,1999,62,352}}]][[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | |Source=Clavulolactones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard.[[Reference:Iwashima_M:Okamoto_K:Konno_F:Iguchi_K:,J. Nat. Prod.,1999,62,352|{{RelationTable/GetFirstAuthor|Reference:Iwashima_M:Okamoto_K:Konno_F:Iguchi_K:,J. Nat. Prod.,1999,62,352}}]][[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | ||
|Chemical Synthesis=Clavulolactone III was converted from clavulone III.[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | |Chemical Synthesis=Clavulolactone III was converted from clavulone III.[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | ||
Revision as of 19:00, 25 February 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR8036 |
| LipidMaps | LMFA03120017 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406CV02 |
| clavulolactone III | |
|---|---|
| |
| Structural Information | |
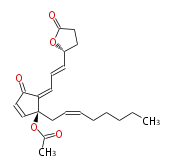
| (R) -4-{ (1E,3Z) -3- [ (S) -2-acetoxy-2- [ (Z) -2-octenyl ] -5-oxo-3-cyclopentenylidene ] -1-propenyl}-4-butanolide | |
| |
| Formula | C22H28O5 |
| Exact Mass | 372.193674006 |
| Average Mass | 372.45472000000007 |
| SMILES | CC(=O)O[C@@](CC=CCCCCC)(C=2)C(C(C2)=O)=CC=C[C@H](O1)CCC(=O)1 |
| Physicochemical Information | |
| [ α ]D -7.3°(C 0.21, CHCl3) IguchiKet al. | |
| Clavulolactones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard. Iwashima_M et al. Iguchi_K et al. | |
| Clavulolactone III was converted from clavulone III. Iguchi_K et al. | |
| Spectral Information | |
| Mass Spectra | EIMS m/z 372 (M+). HREIMS m/z 372.1961 for C22}H_{28O5, calcd 372.1937. IguchiKet al. |
| UV Spectra | λ EtOH max 293 nm( ε 15500),229 nm( ε 17900) IguchiKet al. |
| IR Spectra | ν film max 1778,1742, 1698, 1643, and 1232cm-1 IguchiKet al. |
| NMR Spectra | 1H-NMR(400MHz,CDCl3) δ ppm0.88(3H,t,J=7.1Hz),1.22-1.33(6H,m),1.97(2H,brq,J=7.2Hz),2.03(3H,s),2.05(1H,m),2.47(1H,m),2.58(2H,dd,J=6.9,9.3Hz),2.65(1H,brdd,J=7.6,14.3Hz),2.84(1H,brdd,J=7.3,14.3Hz),5.10(1H,brdd,J=7.3,7.9Hz),5.22(1H,m),5.54(1H,m),6.08(1H,dd,J=7.3,15.6Hz),6.38(1H,d,J=6.1Hz),6.53(1H,d,J=11.3Hz),7.49(1H,d,J=6.1Hz),7.82(1H,brdd,J=11.3,15.6Hz). IguchiKet al. 13C-NMR(100MHz,CDCl3) δ ppm14.0,21.6,22.5,27.4,28.6,28.6,29.0,31.4,35.7,80.0,85.0,121.1,127.6,132.4,135.0,136.6,139.8,139.8,156.3,169.6,176.5,194.0. IguchiKet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|