LBF20406HO21: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=- | |LipidMaps=- | ||
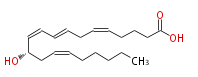
|SysName=12 (S) -Hydoxy-5,8,10,14- (Z,E,Z,Z) -eicosatetraenoic acid | |SysName=12 (S) -Hydoxy-5,8,10,14- (Z,E,Z,Z) -eicosatetraenoic acid | ||
|Reflactive=METHYL ESTER ; [<FONT FACE="Symbol">a</FONT>]X<sub>D</sub><sup>25</sup>=+1.50°(C=0.2, CHLOROFORM) | |Reflactive=METHYL ESTER ; [<FONT FACE="Symbol">a</FONT>]X<sub>D</sub><sup>25</sup>=+1.50°(C=0.2, CHLOROFORM) [[Reference:Corey_EJ:Niwa_H:Knolle_J:,J. Am. Chem. Soc.,1978,100,1942|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Niwa_H:Knolle_J:,J. Am. Chem. Soc.,1978,100,1942}}]], [<FONT FACE="Symbol">a</FONT>]X<sub>D</sub><sup>22</sup>=+13°(C=1.5, ACETONE) [[Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789|{{RelationTable/GetFirstAuthor|Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789}}]] | ||
|Solubility=DIETHYL ETHER , ACETONE , BENZENE | |Solubility=DIETHYL ETHER , ACETONE , BENZENE [[Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789|{{RelationTable/GetFirstAuthor|Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789}}]] | ||
|Mass Spectra=METHYL ESTER ; m/e 316, 303, 223, 191, 141, 107(base peak) | |Mass Spectra=METHYL ESTER ; m/e 316, 303, 223, 191, 141, 107(base peak) [[Reference:Just_G:Wang_ZY:,J. Org. Chem.,1986,51,4796|{{RelationTable/GetFirstAuthor|Reference:Just_G:Wang_ZY:,J. Org. Chem.,1986,51,4796}}]] | ||
|UV Spectra=METHYL ESTER ; <FONT FACE="Symbol">l</FONT> <SUP><FONT SIZE=-1>M</FONT></SUP><SUP><FONT SIZE=-1>e</FONT></SUP><SUP><FONT SIZE=-1>O</FONT></SUP><SUP><FONT SIZE=-1>H</FONT></SUP><SUB><FONT SIZE=-1>m</FONT></SUB><SUB><FONT SIZE=-1>a</FONT></SUB><SUB><FONT SIZE=-1>x</FONT></SUB> = 234nm | |UV Spectra=METHYL ESTER ; <FONT FACE="Symbol">l</FONT> <SUP><FONT SIZE=-1>M</FONT></SUP><SUP><FONT SIZE=-1>e</FONT></SUP><SUP><FONT SIZE=-1>O</FONT></SUP><SUP><FONT SIZE=-1>H</FONT></SUP><SUB><FONT SIZE=-1>m</FONT></SUB><SUB><FONT SIZE=-1>a</FONT></SUB><SUB><FONT SIZE=-1>x</FONT></SUB> = 234nm [[Reference:Just_G:Wang_ZY:,J. Org. Chem.,1986,51,4796|{{RelationTable/GetFirstAuthor|Reference:Just_G:Wang_ZY:,J. Org. Chem.,1986,51,4796}}]]METHYL ESTER ; <FONT FACE="Symbol">l</FONT> <SUP><FONT SIZE=-1>E</FONT></SUP><SUP><FONT SIZE=-1>t</FONT></SUP><SUP><FONT SIZE=-1>O</FONT></SUP><SUP><FONT SIZE=-1>H</FONT></SUP><SUB><FONT SIZE=-1>m</FONT></SUB><SUB><FONT SIZE=-1>a</FONT></SUB><SUB><FONT SIZE=-1>x</FONT></SUB> = 237nm(<FONT FACE="Symbol">e</FONT> 30500) [[Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400}}]] | ||
|IR Spectra=NEAT : <FONT FACE="Symbol">n</FONT> 3480b, 1710, 1460, 1400cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | |IR Spectra=NEAT : <FONT FACE="Symbol">n</FONT> 3480b, 1710, 1460, 1400cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789|{{RelationTable/GetFirstAuthor|Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(250MHz, ACETONE-D<SUB><FONT SIZE=-1>6</FONT></SUB>) ; <FONT FACE="Symbol">d</FONT> 6.58(dd, J=15.3, 11.0Hz, 1H, 10-CH), 5.97(t, J=11.0Hz, 1H, 9-CH), 5.72(dd, J=15.3, 6.2 Hz, 1H, 11-CH), 5.29(m, 5H, 5,6,8,14,15-CH), 4.16(q, J=6.3Hz, 1H, 12-CH), 2.94(t, J=6.1Hz, 2H, 7-CH), 2.27(t,J= 7.4Hz, 2H,2-CH), 2.22(m, 2H, 13-CH), 1.66 and 2.14(m, 2H, 4-and 16-CH), 0.87(t, J=6.3Hz, 3H, 20-CH) | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(250MHz, ACETONE-D<SUB><FONT SIZE=-1>6</FONT></SUB>) ; <FONT FACE="Symbol">d</FONT> 6.58(dd, J=15.3, 11.0Hz, 1H, 10-CH), 5.97(t, J=11.0Hz, 1H, 9-CH), 5.72(dd, J=15.3, 6.2 Hz, 1H, 11-CH), 5.29(m, 5H, 5,6,8,14,15-CH), 4.16(q, J=6.3Hz, 1H, 12-CH), 2.94(t, J=6.1Hz, 2H, 7-CH), 2.27(t,J= 7.4Hz, 2H,2-CH), 2.22(m, 2H, 13-CH), 1.66 and 2.14(m, 2H, 4-and 16-CH), 0.87(t, J=6.3Hz, 3H, 20-CH) [[Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789|{{RelationTable/GetFirstAuthor|Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789}}]]. <SUP><FONT SIZE=-1>1</FONT></SUP><SUP><FONT SIZE=-1>3</FONT></SUP>NMR(C<SUB><FONT SIZE=-1>6</FONT></SUB>D<SUB><FONT SIZE=-1>6</FONT></SUB>) : 174.3, 137.77, 132.02, 129.98, 129.62, 128.88, 128.69, 125.96, 124.44 [[Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789|{{RelationTable/GetFirstAuthor|Reference:Leblanc_Y:Fitzsimmons_BJ:Adams_J:Perez_F:Rokach_J:,J. Org. Chem.,1986,51,789}}]] | ||
}} | }} | ||
Revision as of 09:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR6102 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406HO21 |
| GlcNAca/b1-3Xyla-4Galb1-3GalNAca1-4(NeuAc?1-2NeuGc4Mea1-3)GalNAcb1-4(EtnP-6)GlcNAcb1-3Manb1-4Glcb1-1Cer | |
|---|---|
| |
| Structural Information | |
| 12 (S) -Hydoxy-5,8,10,14- (Z,E,Z,Z) -eicosatetraenoic acid | |
| Formula | C20H32O3 |
| Exact Mass | 320.23514489 |
| Average Mass | 320.46628 |
| SMILES | C(CC=CC[C@@H](C=CC=CCC=CCCCC(O)=O)O)CCC |
| Physicochemical Information | |
| DIETHYL ETHER , ACETONE , BENZENE LeblancYet al. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER ; m/e 316, 303, 223, 191, 141, 107(base peak) JustGet al. |
| UV Spectra | METHYL ESTER ; l MeOHmax = 234nm JustGet al.METHYL ESTER ; l EtOHmax = 237nm(e 30500) HambergMet al. |
| IR Spectra | NEAT : n 3480b, 1710, 1460, 1400cm-1 LeblancYet al. |
| NMR Spectra | 1H-NMR(250MHz, ACETONE-D6) ; d 6.58(dd, J=15.3, 11.0Hz, 1H, 10-CH), 5.97(t, J=11.0Hz, 1H, 9-CH), 5.72(dd, J=15.3, 6.2 Hz, 1H, 11-CH), 5.29(m, 5H, 5,6,8,14,15-CH), 4.16(q, J=6.3Hz, 1H, 12-CH), 2.94(t, J=6.1Hz, 2H, 7-CH), 2.27(t,J= 7.4Hz, 2H,2-CH), 2.22(m, 2H, 13-CH), 1.66 and 2.14(m, 2H, 4-and 16-CH), 0.87(t, J=6.3Hz, 3H, 20-CH) LeblancYet al.. 13NMR(C6D6) : 174.3, 137.77, 132.02, 129.98, 129.62, 128.88, 128.69, 125.96, 124.44 LeblancYet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|