LBF20406OX02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Common Name=&&12-oxo-5Z,8Z,10E,14Z-eicosatetraenoic acid&& | |Common Name=&&12-oxo-5Z,8Z,10E,14Z-eicosatetraenoic acid&& | ||
|UV Spectra=<FONT FACE="Symbol">l</FONT>max: 280nm <FONT FACE="Symbol">e</FONT>: 30,000 | |UV Spectra=<FONT FACE="Symbol">l</FONT>max: 280nm <FONT FACE="Symbol">e</FONT>: 30,000 | ||
|Source=12-OxoETE is synthesized by human platelets and Aplaysia nervous tissue after incubation with arachidonic acid [[Reference:Falgueyret_JP:Leblanc_Y:Riendeau_D:,FEBS Lett.,1990,262,197|{{RelationTable/GetFirstAuthor|Reference:Falgueyret_JP:Leblanc_Y:Riendeau_D:,FEBS Lett.,1990,262,197}}]][[Reference:Fruteau_de_Laclos_B:Maclouf_J:Poubelle_P:Borgeat_P:,Prostaglandins,1987,33,315|{{RelationTable/GetFirstAuthor|Reference:Fruteau_de_Laclos_B:Maclouf_J:Poubelle_P:Borgeat_P:,Prostaglandins,1987,33,315}}]];>. Microsomal fractions of various tissues will reduce 12-oxoETE to 12(S)-HETE or a mixture of 12(S)- and 12(R)-HETE [[Reference:Falgueyret_JP:Leblanc_Y:Riendeau_D:,FEBS Lett.,1990,262,197|{{RelationTable/GetFirstAuthor|Reference:Falgueyret_JP:Leblanc_Y:Riendeau_D:,FEBS Lett.,1990,262,197}}]][[Reference:Falgueyret_JP:Leblanc_Y:Rokach_J:Riendeau_D:,Biochem. Biophys. Res. Commun.,1988,156,1083|{{RelationTable/GetFirstAuthor|Reference:Falgueyret_JP:Leblanc_Y:Rokach_J:Riendeau_D:,Biochem. Biophys. Res. Commun.,1988,156,1083}}]];>. | |||
|Chemical Synthesis= | |||
|Metabolism= | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 07:00, 25 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8157 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406OX02 |
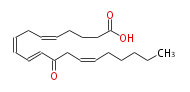
| 12-oxo-5Z,8Z,10E,14Z-eicosatetraenoic acid | |
|---|---|
| |
| Structural Information | |
| 12-oxo-5Z,8Z,10E,14Z-eicosatetraenoic acid | |
| |
| Formula | C20H30O3 |
| Exact Mass | 318.21949482599996 |
| Average Mass | 318.4504 |
| SMILES | C(CC=CCC(C=CC=CCC=CCCCC(O)=O)=O)CCC |
| Physicochemical Information | |
| 12-OxoETE is synthesized by human platelets and Aplaysia nervous tissue after incubation with arachidonic acid Falgueyret_JP et al. Fruteau_de_Laclos_B et al.;>. Microsomal fractions of various tissues will reduce 12-oxoETE to 12(S)-HETE or a mixture of 12(S)- and 12(R)-HETE Falgueyret_JP et al. Falgueyret_JP et al.;>. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | lmax: 280nm e: 30,000 |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|