LBF22506SC01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Common Name=&&4, 7, 10, 13, 16-Docosapentaenoic acid&& | |Common Name=&&4, 7, 10, 13, 16-Docosapentaenoic acid&& | ||
|Solubility=soluble in chloroform, heptane and methyi alcohol. | |Solubility=soluble in chloroform, heptane and methyi alcohol. | ||
|Source=Brain phosphatides. | |||
|Chemical Synthesis= | |||
|Metabolism=The synthesis of 4,7,10,13,16-22:5 occurs via the following reaction sequence [[Reference:Sprecher_H:Luthria_DL:Mohammed_BS:Baykousheva_SP:,J. Lipid. Res.,1995,36,2471|{{RelationTable/GetFirstAuthor|Reference:Sprecher_H:Luthria_DL:Mohammed_BS:Baykousheva_SP:,J. Lipid. Res.,1995,36,2471}}]];>: 9,12-18:2 --> 6,9,12-18:3 --> 8,11,14-20:3 --> 5,8,11,14-20:4 --> 7,10,13,16-22:4 --> 9,12,15,18-24:4 --> 6,9,12,15,18-24:5 --> 4,7,10,13,16-22:5. According to these pathways the 24-carbon acids that are made in the endoplasmic reticulum move to a site for partial beta-oxidation, which is most likely peroxisomes. | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 07:00, 25 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA0221 |
| LipidMaps | LMFA01030182 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF22506SC01 |
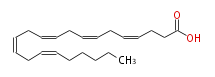
| 4, 7, 10, 13, 16-Docosapentaenoic acid | |
|---|---|
| |
| Structural Information | |
| 4, 7, 10, 13, 16-Docosapentaenoic acid | |
| |
| Formula | C22H34O2 |
| Exact Mass | 330.255880332 |
| Average Mass | 330.50416000000007 |
| SMILES | C(CCC(O)=O)=CCC=CCC=CCC=CCC=CCCCCC |
| Physicochemical Information | |
| soluble in chloroform, heptane and methyi alcohol. | |
| Brain phosphatides. | |
| The synthesis of 4,7,10,13,16-22:5 occurs via the following reaction sequence Sprecher_H et al.;>: 9,12-18:2 --> 6,9,12-18:3 --> 8,11,14-20:3 --> 5,8,11,14-20:4 --> 7,10,13,16-22:4 --> 9,12,15,18-24:4 --> 6,9,12,15,18-24:5 --> 4,7,10,13,16-22:5. According to these pathways the 24-carbon acids that are made in the endoplasmic reticulum move to a site for partial beta-oxidation, which is most likely peroxisomes. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|