LBF22603SC01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
|Solubility=soluble in benzene, chloroform, methyl alcohol, ether and petroleum ether.[[Reference:Klenk_E:Bongard_W:,Hoppe Seylers Z. Physiol. Chem.,1952,291,104|{{RelationTable/GetFirstAuthor|Reference:Klenk_E:Bongard_W:,Hoppe Seylers Z. Physiol. Chem.,1952,291,104}}]][[Reference:Klenk_E:Lindlar_F:,Hoppe Seylers Z. Physiol. Chem.,1955,299,74|{{RelationTable/GetFirstAuthor|Reference:Klenk_E:Lindlar_F:,Hoppe Seylers Z. Physiol. Chem.,1955,299,74}}]][[Reference:Whitcutt_JM:,Biochem. J.,1957,67,60|{{RelationTable/GetFirstAuthor|Reference:Whitcutt_JM:,Biochem. J.,1957,67,60}}]] | |Solubility=soluble in benzene, chloroform, methyl alcohol, ether and petroleum ether.[[Reference:Klenk_E:Bongard_W:,Hoppe Seylers Z. Physiol. Chem.,1952,291,104|{{RelationTable/GetFirstAuthor|Reference:Klenk_E:Bongard_W:,Hoppe Seylers Z. Physiol. Chem.,1952,291,104}}]][[Reference:Klenk_E:Lindlar_F:,Hoppe Seylers Z. Physiol. Chem.,1955,299,74|{{RelationTable/GetFirstAuthor|Reference:Klenk_E:Lindlar_F:,Hoppe Seylers Z. Physiol. Chem.,1955,299,74}}]][[Reference:Whitcutt_JM:,Biochem. J.,1957,67,60|{{RelationTable/GetFirstAuthor|Reference:Whitcutt_JM:,Biochem. J.,1957,67,60}}]] | ||
|Chromatograms=Gas liquid chromatogram {{Image200|LBF22603SC01CH0002.gif}} (provided by Dr. Akiko Horiuchi). | |Chromatograms=Gas liquid chromatogram {{Image200|LBF22603SC01CH0002.gif}} (provided by Dr. Akiko Horiuchi). | ||
|Source=South African pilchard oil; brain phosphatides; liver lipids of cattle. It is found mainly in fish and marine microorganisms and plants. DHA is also an essential component of the brain, eyes, and other nervous system tissues. Tuna eyeballs are one of the major source . | |||
|Chemical Synthesis= | |||
|Metabolism=Metabolic product of <FONT FACE="Symbol">a</FONT>-linolenic acid (9,12,25-18:3). The synthesis of 4,7,10,13,16,19-22:6 (=DHA) occurs via the following reaction sequence [[Reference:Sprecher_H:Luthria_DL:Mohammed_BS:Baykousheva_SP:,J. Lipid. Res.,1995,36,2471|{{RelationTable/GetFirstAuthor|Reference:Sprecher_H:Luthria_DL:Mohammed_BS:Baykousheva_SP:,J. Lipid. Res.,1995,36,2471}}]];>: 9,12,15-18:3 --> 6,9,12,15-18:4 --> 8,11,14,17-20:4 --> 5,8,11,14,17-20:5 --> 7,10,13,16,19-22:5 --> 9,12,15,18,21-24:5 --> 6,9,12,15,18,21-24:6 --> 4,7,10,13,16,19-22:6. According to these pathways the 24-carbon acids that are made in the endoplasmic reticulum move to a site for partial beta-oxidation, which is most likely peroxisomes. | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 07:00, 25 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA0224 |
| LipidMaps | LMFA01030185 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF22603SC01 |
| Docosahexaenoic acid | |
|---|---|
| |
| Structural Information | |
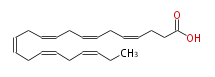
| cis-4, cis-7, cis-10, cis-13, cis-16, cis-19-Docosahexaenoic acid | |
| |
| Formula | C22H32O2 |
| Exact Mass | 328.240230268 |
| Average Mass | 328.48828000000003 |
| SMILES | C(CCC(O)=O)=CCC=CCC=CCC=CCC=CCC=CCC |
| Physicochemical Information | |
| -44.2 to -44.1 °C | |
| 1.5017 at 26 °C | |
| soluble in benzene, chloroform, methyl alcohol, ether and petroleum ether. KlenkEet al. KlenkEet al. Whitcutt_JM | |
| South African pilchard oil; brain phosphatides; liver lipids of cattle. It is found mainly in fish and marine microorganisms and plants. DHA is also an essential component of the brain, eyes, and other nervous system tissues. Tuna eyeballs are one of the major source . | |
| Metabolic product of a-linolenic acid (9,12,25-18:3). The synthesis of 4,7,10,13,16,19-22:6 (=DHA) occurs via the following reaction sequence Sprecher_H et al.;>: 9,12,15-18:3 --> 6,9,12,15-18:4 --> 8,11,14,17-20:4 --> 5,8,11,14,17-20:5 --> 7,10,13,16,19-22:5 --> 9,12,15,18,21-24:5 --> 6,9,12,15,18,21-24:6 --> 4,7,10,13,16,19-22:6. According to these pathways the 24-carbon acids that are made in the endoplasmic reticulum move to a site for partial beta-oxidation, which is most likely peroxisomes. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | Gas liquid chromatogram 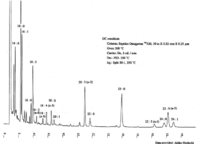 (provided by Dr. Akiko Horiuchi). |