Category:LBSG
Sialic acid シアル酸
Sialic acid is a collective noun and comprises a family of derivatives of neuraminic acid (5-amino- 3,5-dideoxy- D-glycero- D-galacto- nonulosonic acid)[1]. Nowadays, there are over 30 derivatives of neuraminic acid, including:
|
シアル酸は、ノイラミン酸(5-アミノ-3,5-ジデオキシ-D-グリセロ-D-ガラクト-ノヌロン酸)に由来する物質の総称です。 現在、ノイラミン酸には以下を含む30 種以上の誘導体が知られています。
|
History
|
|
- ↑ Schauer R "Sialic Acids - Chemistry, Metabolism and Function" Springer-Verlag, 1982.ISBN 978-3-7091-8680-0
- ↑ Lundblad A "Gunnar Blix and his discovery of sialic acids. Fascinating molecules in glycobiology" Ups J Med Sci. 2015; 120:104–112 PMID 25921326
- ↑ Gottschalk A. "Structural Relationship between Sialic Acid, Neuraminic Acid and 2-Carboxy-Pyrrole" Nature 1955; 176, 881 - 882 doi:10.1038/176881a0
- ↑ Kanfer JN and Hakomori S "Sphingolipid biochemistry (Handbook of Lipid Research 3)" Chapter 1, Springer Science & Business Media, 2012 ISBN 978-1475703986
Ganglioside ガングリオシド
Ganglioside is a glycosphingolipid with one or more sialic acid. It was named by Klenk E. in 1942 as the major sphingolipid in ganglion, a nerve cell cluster.[1] In vertebrate, close to 190 gangliosides have been identified.[2] They reside on lipid rafts of cell membranes, and involve in many cellular functions. Major structures with sialic acids include ganglio, lacto, and neolacto series. |
酸性糖であるシアル酸を含む糖脂質をガングリオシドと呼びます。神経細胞の集まりである神経核 (ganglion) に多いことから Klenk E.により1942年に命名されました。 脊椎動物では現在およそ190種のガングリオシドが見出されています。ガングリオシドは細胞膜の脂質ラフトに存在し、多くの生理機能に関わっています。シアル酸の結合する主要系列はガングリオ、ラクト、ネオラクト等です。 |
- ↑ Sweeley CC, Siddiqui B. "Chemistry of Mammalian Glycolipids" Chapter 6 in The Glycoconjugates Volume I: Mammalian Glycoproteins and Glycolipids (Ed. Horowitz MI, Pigman W) 1977 Elsevier Inc. ISBN 978-0-12-356101-5
- ↑ Yu RK, Tsai YT, Ariga T, Yanagisawa M. (2011) Structures, biosynthesis, and functions of gangliosides--an overview" J Oleo Sci. 60(10):537-44. PMID 21937853
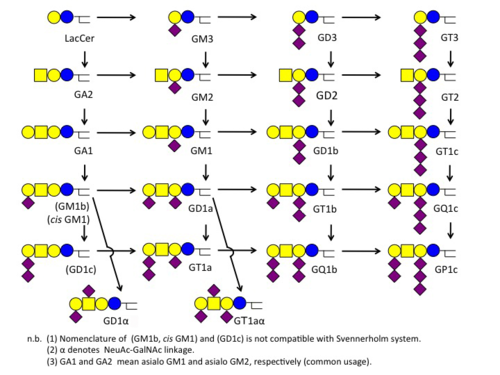
Svennerholm Notation スヴェンネルホルム表記
The notation was proposed by the Swedish scientist, Lars Svennerholm. Each alpha-numeric digit stands for the number of sialic acids or sugars. |
スウェーデンの科学者 Lars Svennerholm が導入した記法です。各桁の数字やアルファベットはシアル酸の数や糖の数を意味します。 |
| Abbreviation | GM | GD | GT | GQ | GP |
|---|---|---|---|---|---|
| Number of Sialic Acid | 1 | 2 | 3 | 4 | 5 |
| Suffix Number | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Core | Gg4Cer | Gg3Cer | LacCer | GalCer |
| Suffix Alphabet (only for Gg4Cer core) | a | b | c |
|---|---|---|---|
| Number of Sialic Acid Attached to IIGal | 1 | 2 | 3 |
- Examples
- GQ1b ... 4 sialic acids (GQ), 4 sugars (GQ1), 2 sialic acids attached to the 2nd galactose (GQ1b)
- GM2 ... 1 sialic acids (GM), 3 sugars (GM2).
Sulfatide スルファチド
More than ten compounds of sulfoglycolipids (glycosphingolipid sulfate, sulfatide, SM4) are identified by differences in sulfonated sugar chains. Among them 3-O-Sulfated galactosylceramide(GalCer) is called galactosylsulfatide, and is present in myelin sheath like GalCer. [1] The existence of glucosylsufatide, 3-O-sulfated glucosylceramide was reported in rat kidney.[2] Sulfatides are present in various organs, such as nervous system, kidney, respiratory organ and digestive tract. The physiological functions of sulfatides are related to myelinogenesis, diabetes, immune system, thrombosis, bacterial and virus infection. [3] Lysosomal storage disease, metachromatic leukodystrophy, is a congenital metabolic disorder, and is caused by deficiency of lysosomal enzyme arylsulfatase A or its activator.[4] |
硫酸化糖脂質には硫酸化される糖鎖の違いにより十数種類が同定されています。その中でもガラクトシルセラミド(GalCer)のガラクトース3位の水酸基に硫酸基を導入したものをガラクトシルスルファチドと呼び、GalCerと同様にミエリンに多く存在します。ラット腎臓にはグルコシルセラミドに硫酸基が結合したグルコシルスルファチドも報告があります。 スルファチドは神経系、腎臓、呼吸器、消化器などさまざまな組織に存在する糖脂質で、ミエリン形成に関わるほか、糖尿病、免疫系、血栓形成、細菌感染、ウィルス感染など生理作用は多岐にわたります。
|
Metabolic pathway[5]
- Biosynthesis
- Cerebroside sulfotransferase (CST) + 3’-phosphoadenosine-5’-phosphosulfate(PAPS)
- Galactosylceramide → galactosylsulfatide
- Degradation
- Arylsulfatase A (ASA) + Saposin B (activator)
- Galctoslsulfatide → Galactosylceramide
- ↑ Ishizuka, I. “Chemistry and functional distribution of sulfoglycolipids” Prog. Lipid Res.1997; 36:245-319 PMID 9640458
- ↑ Iida N, Toida T, Kushi Y, Handa S, Fredman P, Svennerholm L, Ishizuka I. A sulfated glucosylceramide from rat kidney. J Biol Chem. 1989 5;264:5974-80.PMID 2925645
- ↑ Takahashi T, Suzuki T. “Role of sulfatide in normal and pathological cells and tissues” J Lipid Res. 2012; 53: 1437–1450, PMID 22619219 Takahashi T, Suzuki T. “Role of sulfatide in influenza A virus replication” Biol Pharm Bull. 2015; 38:809-16.PMID 26027821
- ↑ Mirzaian M, Kramer G, Poorthuis BJ. “Quantification of sulfatides and lysosulfatides in tissues and body fluids by liquid chromatography-tandem mass spectrometry”J Lipid Res. 2015; 56:936-43. PMID 25632048
- ↑ Honke K. Biosynthesis and biological function of sulfoglycolipids. Proc Jpn Acad Ser B Phys Biol Sci. 2013 89:129-38. PMID 23574804
This category currently contains no pages or media.