LBF20207PG23
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1401 |
| LipidMaps | LMFA03010003 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG23 |
| Prostaglandin E2 | |
|---|---|
| |
| Structural Information | |
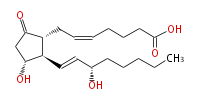
| 7- [ 3R -Hydroxy-2R - (3S -hydroxy-1-trans-octenyl-5-oxocyclopentan-1R -yl ] -5-cis-heptenoic acid | |
| |
| PGE2 | |
| Formula | C20H32O5 |
| Exact Mass | 352.224974134 |
| Average Mass | 352.46508 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]1CC=CCCCC(O)=O)[C@@H](CC1=O)O)CC |
| Physicochemical Information | |
| 65-66°C Corey_EJ et al. | |
| [ α ]26 D =-61.0°(C=1.0, TETRAHYDROFURAN) Corey_EJ et al. | |
| ETHYL ACETATE,THF,CHLOROFORM Donaldson_RE et al. Sih_CJ et al.. STABILITIES: to be stable under neutral condition. to decompose to PGA2 under acidic and to PGB2 under basic conditions Karim_SM et al. Pike_JEet al. | |
| Prostaglandin E2 was found to be accummulating in human semen in an amount of about 13 microgram per ml Bergstrom_S . In most animal tissues prostanoids are synthesized enzymatically de novo upon physiological and pathological stimulations, and this is also the case of prostaglandin E2. | |
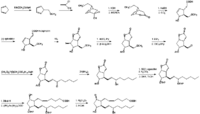 Corey_EJ et al. | |
| Prostaglandin E2 is produced from arachidonic acid via prostaglandins G2 and H2 by the catalyses of prostaglandin endoperoxide synthase (cyclooxygenase) Smith_WL et al. and prostaglandin E synthase Urade_Y et al.. Two isoforms of the cyclooxygnease enzyme responsible for prostaglandin H2 synthesis are present. Cyclooxygenase-1 is constitutively found in most mammalian tissues, while cyclooxygnease-2 is induced rapidly and transiently in physiological and pathological events, especially in inflammation Smith_WL et al.. The biological activities of prostaglandin E2 are lost by oxidation of its 15-hydroxyl group catalyzed by 15-hydroxyprostaglandin dehydrogenase Hansen_HS . | |
| Prostaglandin E2 exhibits various biological activities such as vasodilatation, uterine contraction, gastrointestinal contraction, bronchodilatation, diuresis, pyrexia, inhibition of gastric secretion, bone resorption and immunosuppression Bergstrom_S et al.. Prostaglandin E2 is a ligand to receptors present in the cell membrane, and there are at least 4 subtypes of its receptor. Different tissue distributions and signal transductions of these receptor subtypes explain a variety of biological activities of prostaglandin E2 Negishi_M et al.. | |
| cDNAs of the two cyclooxygenase isozymes responsible for prostaglandin H2 synthesis have been cloned, and the primary structures of these enzymes were deduced from the nucleotide seuences Funk_CD Herschman_HR Their genomic DNA were also cloned, and the genomic structure were eludicated Funk_CD . cDNAs of four subtypes of prostaglandin E2 receptors (EP1-4) were cloned, and the 7-transmembrane domain structures of the receptors coupled with certain G proteins were reported Negishi_M et al.. | |
| Spectral Information | |
| Mass Spectra | d,l-mixture ; 334(M+-18), 316, 298, 190 Chen_SML et al. |
| UV Spectra | |
| IR Spectra | d,l-mixture ; 3400, 1710, 970cm-1 Chen_SML et al. |
| NMR Spectra | 13C-NMR(CDCl3) : 214.71(C9),178.39(C1), 136.62(C14), 131.52(C13), 130.91(C5), 126.69(C6), 73.19(C15), 72.13(C11), 54.55(C12), 53.51(C8), 46.23(C10), 37.00 (C16), 33.56(C2), 31.73(C18), 26.47(C4), 25.20(C7,17), 24.60(C3), 22.64(C19), 14.04. Donaldson_RE et al.. 1H-NMR(CDCl3) : δ 5.67(dd, J=6.6Hz, 15.4, 1H, 14-CH), 5.57(dd, J=8.1, 15.4Hz, 1H, 13-CH), 5.40(m, 2H, 5.6-CH), 4.12(q, J=6.5, 6.7, 6.8Hz, 1H, 15-CH), 4.06(q, J=8.1, 8.2, 8.3Hz, 1H, 11-CH), 2.72(dd, J= Donaldson_RE et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|